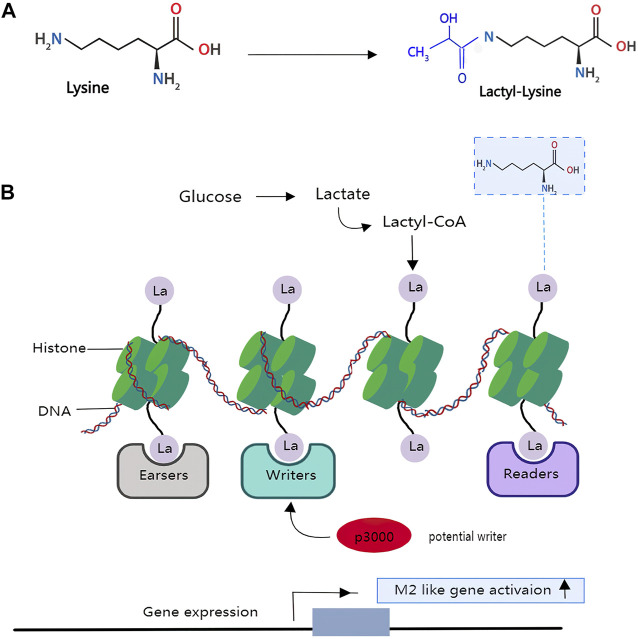
FIGURE 3.
Lactate modifies histones to regulate macrophage polarization and tumour immunity: An achemical modification called lactylation—the addition of a lactyl (La) group to the lysine amino-acid residues in the tails of histone proteins. (A), the structure of lysine and lactyl group. (B), glucose can be incompletely converted to the metabolite lactate in the macrophages or under a hypoxia situation. The lactyl-CoA generated from lactate contributes a lactyl group to the lysine tails of histone proteins via the acetyltransferase enzyme p300 to produce the epigenetic modification called lactyllysine, which allows for gene activation of genes belonging to wound-healing pathways, thus resulting in an M2-like phenotype. However, it is unclear which enzymes generate the intermediate molecule lactyl-CoA, from which La is derived, or which enzymes deposit (writers), remove (erasers) or recognize and interpret (readers) histone lactylation.