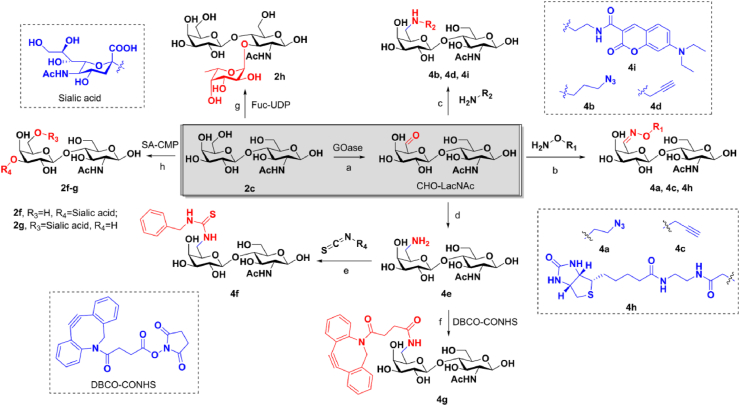
Scheme 1.
Synthesis of LacNAc derivatives. Reagents and conditions: (a) galactose oxidase, HRP, catalase, O2, 50 mmol/L PB, pH 7.0, 30 °C; (b) O-(2-azidoethyl)-hydroxylamine hydrochloride, O-(2-propynylethyl)-hydroxylamine hydrochloride, or biotin-ONH2, pH 7.2, rt; (c) 3-azido-1-propanamine, 2-propynylamine, or coumarin-NH2, NaCNBH3, pH 6.0, 0 °C; (d) NH2OH·HCl, Na2CO3, CH3OH/H2O, rt; NaBH4, NiCl2·6H2O, 0 °C; (e) N-benzylthiourea, pH 8.0, rt; (f) DBCO-CONHS, pH 7.4, rt; (g) GDP-Fuc, α(1,3)-fucosyltransferase; (h) α(2,3)-sialyltransfererase, CMP-sialic acid, Tris-HCl buffer (100 mmol/L, pH 8.0); or α(2,6)-sialyltransferase (Pd2,6ST), CMP-sialic acid, Tris-HCl buffer (100 mmol/L, pH 8.0).