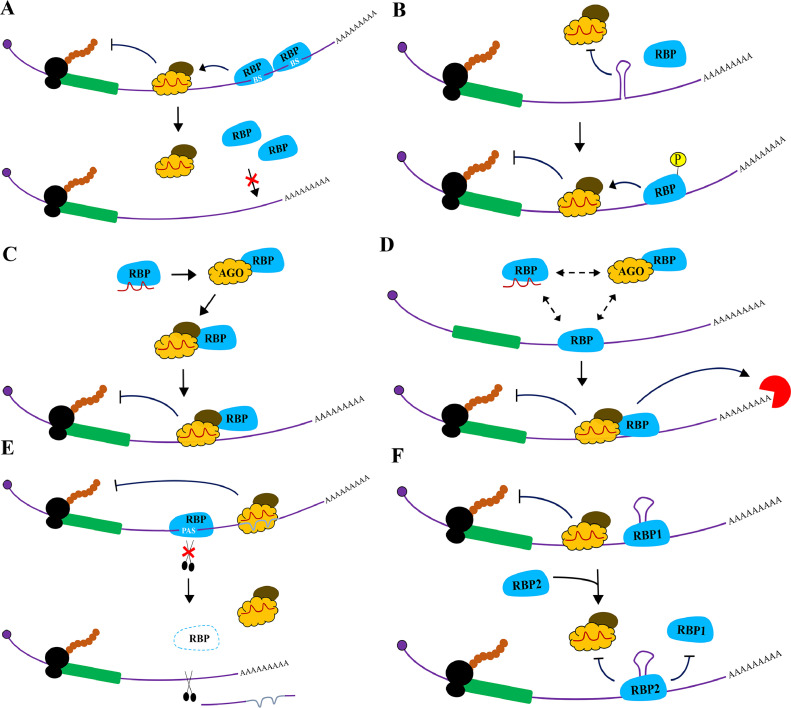
Fig. 2.
Models of co-operative miRNA-AGO: RBP interactions on target mRNAs. (A) The RBP binds multiple sites on the target mRNA and facilitates the interaction between miRNA-AGO and complementary miRNA sequences in the 3′ UTR, thereby cooperating in gene silencing. However, shortening of 3′ UTR results in the loss of RBP binding sites (BS), thus affecting miRNA-AGO association with the target mRNA and its regulation. (B) Structures in the 3′ UTR impede the binding of miRNA-AGO to target sites. Phosphorylated RBPs resolve these secondary structures, thereby allowing the binding of miRNA-AGO to miRNA complementary sites in mRNA 3′ UTRs towards their repression. (C) The RBP assists in the loading of miRNAs into AGO and promotes miRNA-AGO binding to target sites in 3′ UTR for gene silencing. (D) The RBP can simultaneously bind both miRNA and its targets, and its interaction with AGO facilitates the formation of miRISC on respective mRNA 3′ UTRs, thus enabling miRNA-guided decay of targets. (E) The RBP protects Poly(A) Signals (PAS) from cleavage, and loss of its expression leads to deletion of complementary sites for miRNA-AGO binding to the mRNA, resulting in loss of miRNA function. (F) Different RBPs (RBP1 and RBP2) have varied binding affinities towards secondary structures in the 3′ UTR of mRNAs. Depending on their nature of interaction with AGO, they either facilitate the binding of miRNA-AGO to the miRNA complementary sites, leading to target repression or form a stable complex surrounding the secondary structures in 3′ UTR of target mRNAs. Such an arrangement limits the access of miRNA-AGO complex to the target sites embedded in the secondary structures, thus negatively affecting gene silencing. RBPs are presented in Blue, AGO in Yellow, TNRC6 in Brown, Exonucleases in Red, and Ribosomes in Black while the Coding region of the mRNA in Green.