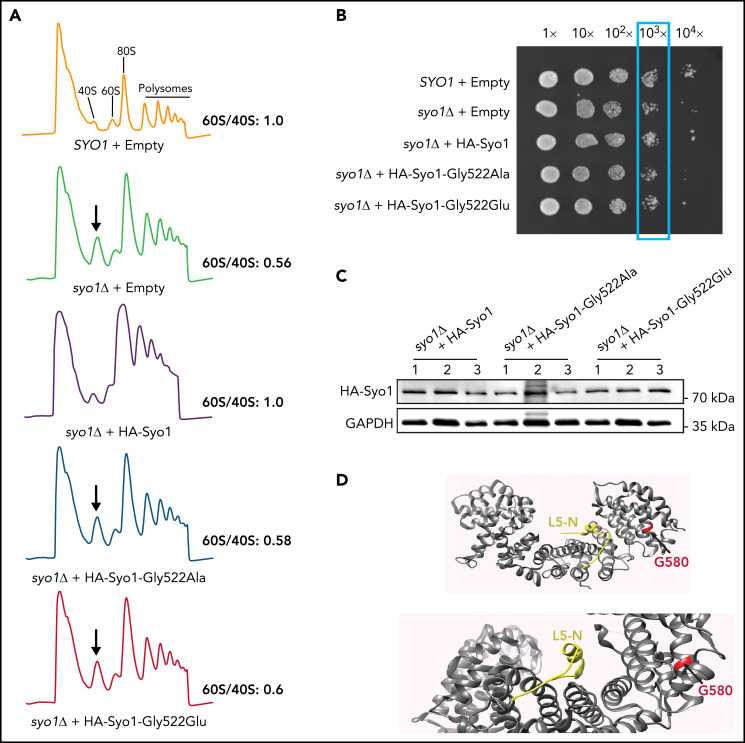
Figure 2.
The HEATR3 Gly584 (yeast Gly522) residue is important for protein function. (A) Polysome profiles of wild-type and syo1Δ yeast cells expressing empty vector controls or HA-tagged Syo1-Gly522 mutations display an altered 60S/40S subunits ratio. Representative examples of a duplicate are shown. (B) Mutations at position Gly522 do not complement yeast growth. The mild growth impairment observed in syo1Δ cells is complemented by a wild-type construct (HA-Syo1) but not by constructs harboring a mutation at position Gly522. The indicated strains were grown at 16°C on synthetic medium lacking leucine for 6 days. (C) The Gly522 mutants are stably expressed in yeast. HA-Syo1 constructs were detected by western blotting with an anti-HA antibody. As loading control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probing was performed. (D) Position of yeast mutation Gly522 on the three-dimensional structure of C thermophilum Syo1 in complex with L5-N (based on PDB 4GMN25). Yeast Gly522 corresponds to Gly584 in the C-terminal (supplemental Figure 1). Two views are shown.