Figure 60.
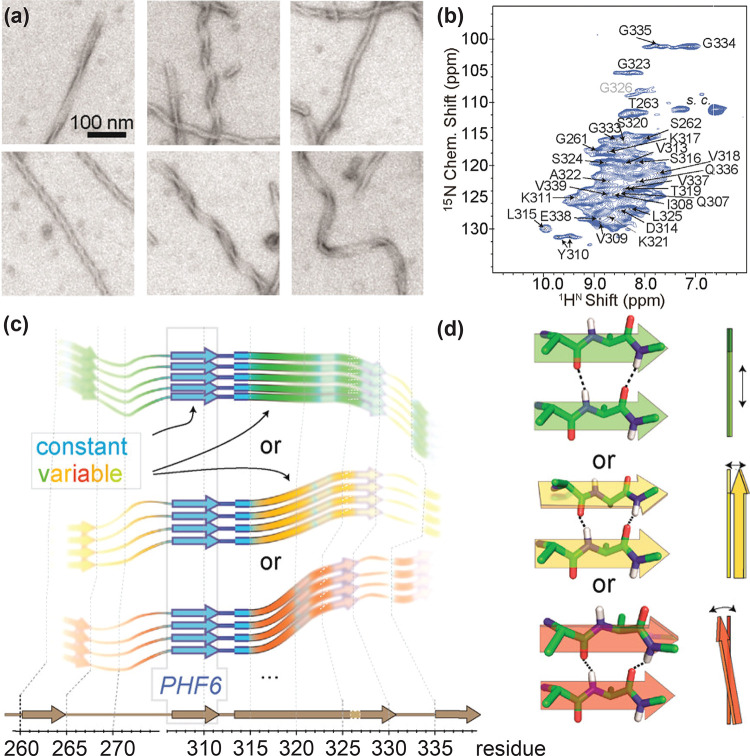
(a) Negative-stain electron microscopy images of heterogeneous paired helical filaments of protein Tau63 and (b) their assigned 1H,15N CP-HSQC spectrum showing significant inhomogeneous line broadening. (c,d) Pictorial interpretation of how the NMR shifts from invariant and variable residues may originate in the fibril building block: (c) secondary structure (β-strand or loop/kink, as derived from 13C secondary chemical shifts) is comparable among different batches of samples; however, 15N chemical shifts of many regions (“non-blue”) vary between different fibrils; (d) variability of the local chemical environment, including H-bond architecture, including shearing (top), twisting (middle), and bending of β-sheets (bottom). Reprinted from ref (63). Copyright 2017 American Chemical Society.