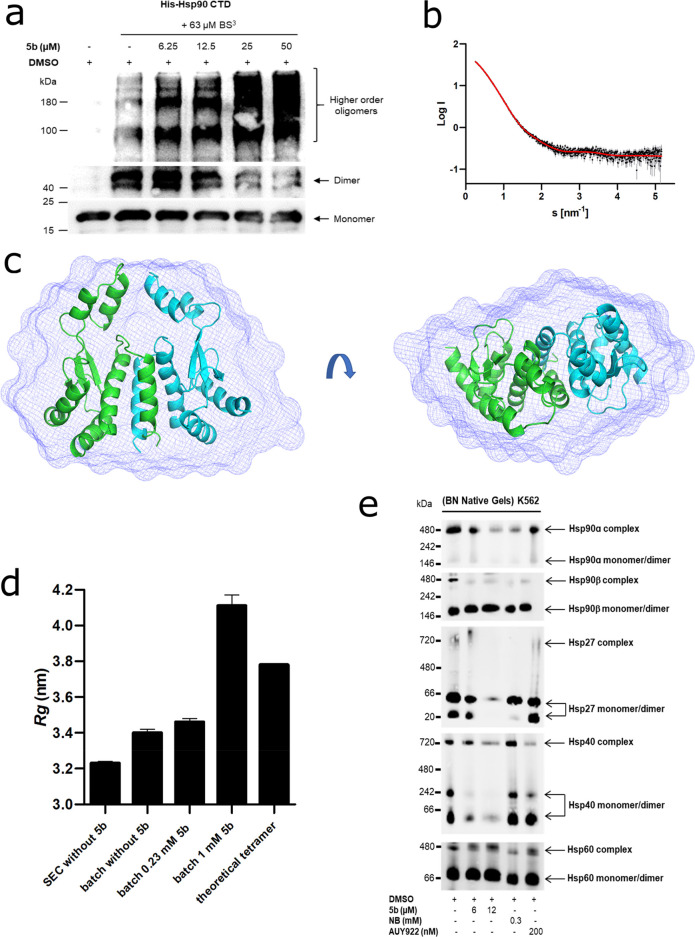
Figure 5.
Effect of 5b on Hsp90 oligomeric species and CTD-mediated dimerization. (a) Recombinant Hsp90α CTD was incubated with 63 μM BS3 cross-linker with (at the indicated concentration) or without 5b, followed by immunoblotting with the anti-Hsp90 (AC88) antibody. (b) The scattering data of Hsp90α CTD is shown in black dots, with gray error bars. The ab initio DAMMIF model fit is shown as a red line. The intensity is displayed as a function of momentum transfer s. (c) The volumetric envelope, calculated from the scattering data using DAMMIF,65 is shown as a blue surface. The monomers of the predicted Hsp90 CTD dimer model are shown in green and cyan. Superimposing was performed using SUPCOMB.65 (d) The radius of gyration (Rg) of the different Hsp90α CTD protein samples was calculated using the Guinier approximation.66 The theoretical Rg of the tetramer was calculated using CRYSOL based on the structure PDB ID 3q6m.67 (e) Native Hsp90 complexes in K-562 (24 h administration of 5b) were identified by running blue native (BN) gels followed by immunoblotting analysis. The cytotoxic concentration of 5b resulted in the potent disruption of Hsp90α, Hsp90β, Hsp40, and Hsp27 complexes and monomers/dimers. AUY922 exposure elevated the expression of HSR associated protein complexes and monomers/dimers (Hsp40 and Hsp27), whereas Hsp60 served as loading controls.