Abstract
Objective
The aim of this meta-analysis was to study the evidence on pain sensitization in knee osteoarthritis (OA), providing a quantitative synthesis of its prevalence and impact. Factors associated with pain sensitization were also investigated.
Methods
Meta-analysis; PubMed (MEDLINE), Cochrane Central Register (CENTRAL), and Web of Science were searched on February 2021. Level I to level IV studies evaluating the presence of pain sensitization in patients with symptomatic knee OA, documented through a validated method (questionnaires or quantitative sensory testing), were included. The primary outcome was the prevalence of pain sensitization. Factors influencing the prevalence were also evaluated, as well as differences in terms of pain thresholds between knee OA patients and healthy controls.
Results
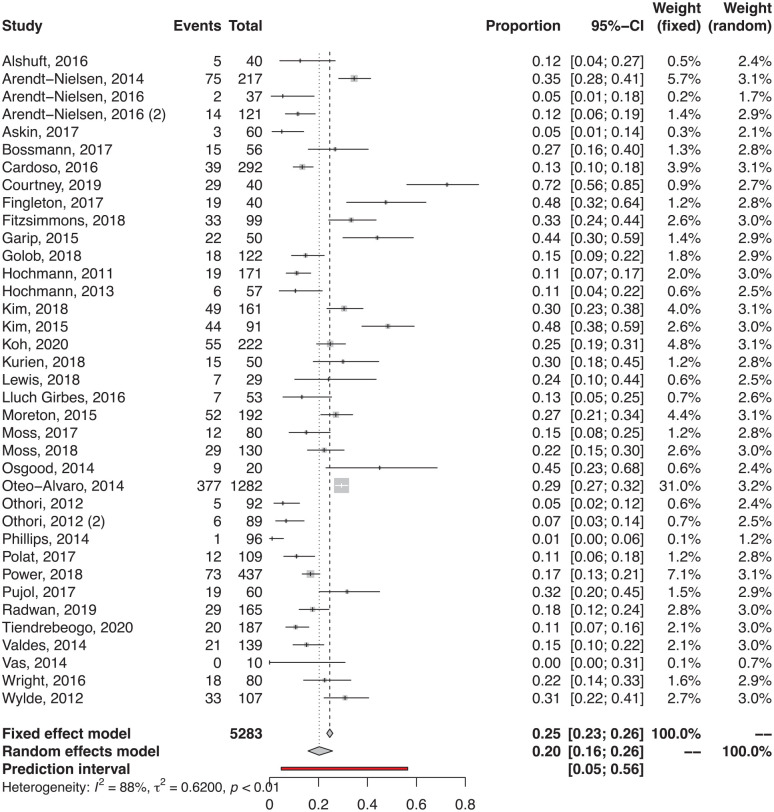
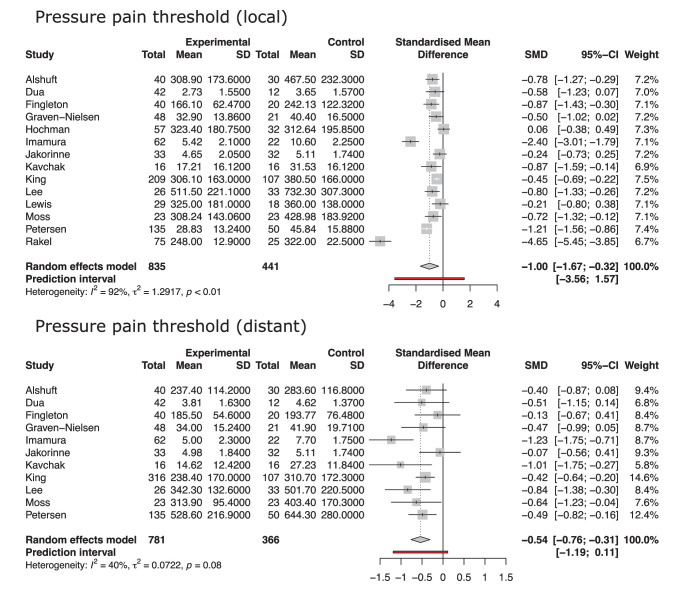
Fifty-three articles including 7,117 patients were included. The meta-analysis of proportion documented a prevalence of pain sensitization of 20% (95% confidence interval [CI] = 16%-26%) with a significant heterogeneity of results (I2 = 89%, P < 0.001). The diagnostic tool used was the main factor influencing the documented prevalence of pain sensitization (P = 0.01). Knee OA patients presented higher pain sensitivity compared with healthy controls, both in terms of local pressure pain threshold (standardized mean difference [SMD] = −1.00, 95% CI = −1.67 to −0.32, P = 0.007) and distant pressure pain threshold (SMD = −0.54, 95% CI = −0.76 to −0.31, P < 0.001).
Conclusions
Knee OA pain presents features that are consistent with a significant degree of pain sensitization. There is a high heterogeneity in the reported results, mainly based on the diagnostic tool used. The identification of the best methods to detect pain sensitization is warranted to correctly evaluate and manage symptoms of patients affected by knee OA.
Registration:
PROSPERO CRD42019123347.
Keywords: knee osteoarthritis, OA, pain, pain sensitization, neuropathic, nociplastic, prevalence
Introduction
Pain is the main symptom of knee osteoarthritis (OA), as well as the most relevant cause of disability and poor quality of life in the affected patients.1,2 Current treatments can only partially address patient symptoms, often offering a limited improvement with persistent pain regardless of the treatment strategy. Structural changes are traditionally considered the trigger of the noxious stimuli and, as such, are the most common target of pain treatment, but the latest studies on this topic led to conflicting results regarding the association between structural damage and pain.3-6 This documented discrepancy could be related to an altered pain perception mechanism, with pain sensitization being increasingly recognized as a key determinant in knee OA–related pain.7-10
Pain sensitization is defined as an altered pain perception caused by increased impulses from peripheral nervous tissues (peripheral sensitization) and/or by the amplification of the pain signals within the central nervous system (central sensitization).11-14 As a consequence, patients present a change in the characteristics of pain including local allodynia and hyperalgesia, as well as in the distribution of pain, as these changes can lead to widespread hypersensitivity, which extends beyond local anatomic changes. 15 The involvement of the nervous system with changes in nervous transmission and cerebrospinal fluid composition affecting level and characteristics of perceived pain leads to the use of the term “neuropathic pain,” to distinguish it from “nociceptive pain” historically considered related to knee OA.16-18 As “neuropathic” pain is a broader concept pertaining different pathologic conditions outside the OA field 19 (i.e., neuropathies, central poststroke pain, etc.) and entailing the presence of a demonstrable neurologic lesion, the term “nociplastic pain” has been introduced to account for the possible involvement of the nervous system in musculoskeletal diseases.20,21 However, neuropathic pain diagnostic tools proved effective in identifying knee OA patients with peripheral and central pain sensitization.22,23 Validated methods such as questionnaires and quantitative sensory testing (QST) protocols evaluating pressure and thermal pain threshold and pain modulating mechanism, such as conditioned pain modulation (CPM) and temporal summation (TS), have been used to investigate the presence of local and widespread pain sensitization in the clinical setting avoiding invasive procedure such as cerebrospinal fluid collection. 24 Although with sometimes controversial findings, recent awareness on this important pain determinant fueled a significant research effort to shed new light in knee OA mechanisms.25,26
The primary aim of this meta-analysis was to provide a quantitative synthesis of the prevalence of pain sensitization in knee OA determining the percentage of patients that present features of pain sensitization according to tools available in the outpatient setting such as questionnaires or QST. The impact of pain sensitization was evaluated comparing pain thresholds—documented with QST—of affected patients and healthy controls. Furthermore, possibly associated factors were investigated through a meta-regression to better identify and manage patients affected by pain sensitization in knee OA.
Materials and Methods
Data Source and Study Selection
After the registration of the protocol on PROSPERO (CRD42019123347), PubMed (MEDLINE), the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science were systematically searched with no time limitation on February 2, 2021, using the following string: (Osteoarthritis OR OA) AND (pain) AND (neuropathic OR nociceptive OR sensitisation OR sensitization OR DN4 OR painDETECT OR S-LANSS OR QST).
After the removal of duplicates, all titles and abstracts were checked to retrieve all eligible articles. Subsequently, if not enough information could be obtained from the abstract, the full-text article was read. Level I to level IV studies on humans reporting the prevalence of pain sensitization in patients with symptomatic knee OA (as a primary or secondary outcome) documented through a validated method (questionnaires or QST), or reporting a comparison of QST between knee OA patients and healthy subjects, were included. Systematic reviews, meta-analyses, narrative reviews, expert opinions, and case reports were excluded. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used. 27 Two authors (D.P., G.C.) independently performed the article selection process, with disagreement on study eligibility solved by a third author (C.C.).
Data Extraction, Study Outcomes, and Quality Assessment
Extracted information on methodology from all eligible studies included level of evidence, study design, technique of pain sensitization assessment, inclusion/exclusion criteria, origin of data, number of patients included, and follow-up length. Information from all eligible studies on characteristics of the study population included sex, age, body mass index (BMI), comorbidities, PROMs (Patient-Reported Outcome Measures), length of symptoms, prevalence of pain sensitization, QST protocol results, and OA stage. Two authors (D.P., G.C.) independently extracted trial information. If data were not available from the published studies, the corresponding authors were contacted. The primary outcome was the prevalence of pain sensitization determined as the percentage of patients that presented features of pain sensitization according to questionnaires or QST. Study and patient characteristics influencing the documented prevalence of pain sensitization in knee OA were also evaluated, as well as differences in terms of pain thresholds—measured with QST—between patients and healthy controls to quantify the impact of pain sensitization in knee OA pain perception.
A previously validated checklist, specifically developed for systematic reviews and meta-analyses addressing prevalence, 28 was used to assess the risk of bias and the quality of the included studies. The evaluation was performed independently by 2 reviewers (D.P., G.C.), and interrater variability was quantified through Cohen’s kappa. Discrepancies were discussed and resolved by a third author (C.C.).
Statistical Analysis
To compute the adequate sample size to detect a prevalence of 20%, the formula of Naing et al. 29 was used and it was determined that at least 246 patients were required. Continuous data were expressed as means and standard deviations and compared as mean differences, whereas binary data were expressed as frequencies and compared as risk ratios. A meta-analysis of proportions was performed to quantify the prevalence of pain sensitization in patients with knee OA with sub-analyses based on the detection method. 30 When a score adopts more than 1 threshold to classify patients (i.e., unlikely, ambiguous, likely), the most conservative class (i.e., likely) was considered in the evaluation of prevalence. Both fixed and random effects were used, with the results of the random effect preferred in case of heterogeneity of the included studies. A linear meta-regression was performed to identify the source of the documented heterogeneity and evaluate the study characteristics influencing the reported prevalence of pain sensitization. Multiple meta-regression, with the variable identified as significantly associated to the documented prevalence, was then performed. Moreover, a meta-analysis was performed to compare the local and distant pressure pain thresholds (PPTs) between knee OA patients and healthy controls. The random effect model with Knapp-Hartung-Sidik-Jonkman adjustment was used, and results were expressed as standardized mean differences (SMDs). The statistical analysis was performed with the packages meta (v4.9-7) and metafor (v2.1-0) in RStudio (v1.2.5019).
Results
Characteristics of the Included Studies and Patients
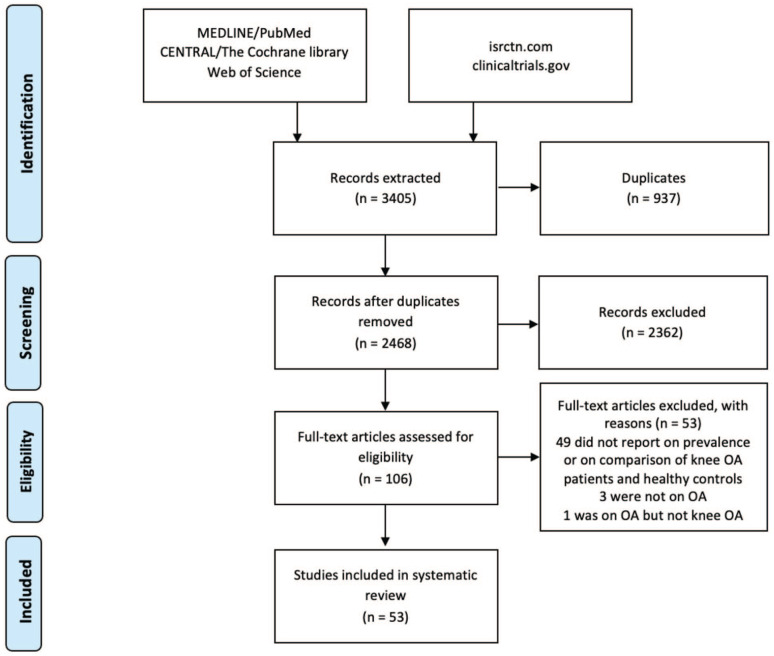
Out of the 3,405 articles retrieved, 53 were selected (Fig. 1).5,22,23,25,26,31-80 All these articles reported pain sensitization prevalence in the included patients, but only 12 studies had the evaluation of the prevalence of pain sensitization in knee OA as a primary aim. Thirty-five studies were focused on other relevant features of pain sensitization, such as its association with patient characteristics and clinical outcomes (19 studies), the detection of pain threshold (5 studies), the effect of a specific treatment on it (5 studies), the development of a new index to evaluate it (3 studies), the evaluation of the different pain phenotypes (2 studies), and the evaluation of the reliability of the QST protocols (1 study). In the remaining 6 studies, pain sensitization was only documented as baseline patient characteristic. All of them used a validated method to detect the presence of pain sensitization in knee OA (some of the studies used more than 1 method to evaluate sensitization): painDETECT questionnaire in 24 studies (4 of which used a modified format), Douleur Neuropathic 4 (DN4) in 4 studies, central sensitization index (CSI) in 3 studies, S-LANSS (Leeds Assessment of Neuropathic Symptoms and Signs) in 2 studies, a newly developed index in 2 studies, and QST in 32 studies. Out of the 32 studies evaluating pain sensitization in knee OA, 10 reported an analysis of the prevalence of pain sensitization. Different methods were used to determine the prevalence of pain sensitization in their samples: Cardoso et al. and Osgood et al. used a cluster analysis of the results of QST protocol; Hochmann et al., Kurien et al., Wright et al., and Wylde et al. created cutoffs based on QST of healthy controls; Bossmann et al. used cutoff values published for healthy subject by the German Research Network on Neuropathic Pain; Lewis et al. considered abnormal a CPM <10% and a TS >10 on a 0 to 100 Visual Analogue Scale; and Courtney et al. and Fingleton et al. considered abnormal a CPM with no change or an increase in pain perception. Patients were recruited from the community in 7 studies, from outpatient settings in 21 studies, from both the community and outpatient settings in 6 studies, from the surgery list in 14 studies, and from both the outpatient settings and the surgery list in 1 study, whereas 4 studies did not report the method of patient recruitment. Overall, 7,117 patients were included, with a male/female ratio ranging from 0 to 3.3, a mean age ranging from 51 to 76, a mean BMI ranging from 25 to 38, and a mean pain duration ranging from 273 days to 11.9 years. Table 1 reports detailed information on studies and patient characteristics.
Figure 1.
PRISMA flowchart of the study selection process. OA = osteoarthritis; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Table 1.
Characteristics of the Included Studies and Patients.
| Study | Origin of Patients | Inclusion Criteria | Exclusion Criteria | Diagnostic Tool | Study Aim | # pts (#Knees) (M, F) | Age, Mean ± SD (Range) | BMI, Mean ± SD (Range) | Pain Duration, Mean ± SD (Range) |
|---|---|---|---|---|---|---|---|---|---|
| Alshuft et al. 31 | Outpatients + community | Radiologic KOA with >3 mo chronic pain | Comorbidities, a age <18, pregnancy, previous TKA or MRI contraindications | PDQ | NeuP b | 40 (19 M, 21 F) | 66.1 ± 8.5 (45.4-83.0) | 28.8 ± 4.9 (20-41.1) | 8.5 (1-38) |
| Arendt-Nielsen et al. 33 | Outpatients + community | ACR KOA criteria, discontinuation of analgesic | Secondary KOA, comorbidities, a pregnancy | New index, QST | NeuP b | 217 (102 M, 115 F) | 63.8 | 28.3 | NR |
| Arendt-Nielsen et al. 32 | Outpatients | ACR KOA criteria, K-L 1-3, worst pain 4-9 analgesic discontinuation | Comorbidities, a allergy to medications, normal ECG and labor, changed FKT (14 d) | PDQ, QST | Oth c | 37 (14 M, 23 F) | 63.3 ± 7.6 | 28.2 ± 4.1 | 10.9 ± 7.5 |
| Arendt-Nielsen et al. (2) 34 | NR | ACR KOA criteria, K-L 1-3, worst pain 4-9, age 40-75, stable knee pain ≥6 mo | Secondary KOA, rheumatic diseases, prior treatment with IA HA (≤24 w) steroid (≤12 w), botulinum toxin (any time) | PDQ, QST | Oth c | 121 (59 M, 62 F) | 62.3 ± 8.6 | 28 ± 3.9 | 8.6 ± 6.6 |
| Askin, et al. 35 | NR | ACR KOA criteria, chronic pain | Previous knee surgery, active inflammation, comorbidities, a pregnant | PDQ, DN4 | NeuP Prev d | 60 (14 M, 46 F) | 56.2 ± 10.0 (32-82) | 29.8 ± 4.8 (20.3-39.5) | 9.1 ± 10.9 |
| Blikman et al. 36 | Outpatients | age >18 y, primary painful KOA (K-L>1) | Comorbidities, a hip/knee surgery (6 mo), no Dutch speakers | mPDQ | NeuP b | 138 (66 M, 72 F) | 61.9 ± 10.5 | 28.7 ± 5.4 | 2.4 ± 0.4 |
| Bossmann et al. 37 | SurgeryList | Painful KOA | Major knee surgery (2 y), comorbidities a | QST | NeuP b | 56 (19 M, 37 F) | 68 ± 9.3 (39-87) | NR | 7.5 |
| Cardoso et al. 38 | Community | Age 45-85 y, African American or non-Hispanic whites. ACR KOA criteria | Daily opioids use, major knee surgery, comorbidities a | QST | NeuP b | 292 (36.5% M, 63.5% F) | 57 | NR | NR |
| Courtney et al. 39 | Outpatients | KOA, K-L ≥II | TKA, ligament deficit, comorbidities a | QST | NeuP b | 40 (13 M, 27 F) | 59.1 ± 8.3 | 36.9 ± 13.5 | 11.9 ± 9.0 |
| Dua et al. 40 | Outpatients | Painful KOA, ACR OA criteria, walk pain ≥20/100, K-L ≥2 | Other painful joints, comorbidities, a TKA | QST | NeuP b | 42 (13 M, 29 F) | 54 ± 8 | NR | NR |
| Fingleton et al. 41 | Outpatients | ACR KOA criteria fulfilled, NRS >3 | Comorbidities, a antidepressant, anticonvulsant TKA, <90° of flexion | QST | NeuP b | 40 (22 M, 18 F) | 63.7 ± 10.3 | NR | NR |
| Fitzsimmons et al. 42 | SurgeryList | >18 y, medically safe to undergo surgery. English speaking | TKA, comorbidities a | S-LANSS | NeuP b | 99 (32 M, 67 F) | 65.1 ± 8.9 | NR | NR |
| Garip et al. 43 | Outpatients | ACR KOA criteria, >1 mo knee pain | Comorbidities a | PDQ | NeuP Prev d | 50 (21 M, 29 F) | 50.9 ± 5.1 (41-65) | NR | NR |
| Graven-Nielsen et al. 45 | Outpatients + SurgeryList | ACR KOA criteria, >3 mo knee pain (VAS >4) | Comorbidities, a no pain medications (48 h) | QST | NeuP | 48 (12 M, 36 F) | 65 (40-86) | NR | 6.6 ± 0.9 |
| Golob et al. 44 | Outpatients | ACR KOA criteria, >12 mo knee pain | Major knee surgery, knee infection, RA | PDQ | NeuP Prev d | 122 (36 M, 86 F) | 64.6 | NR | NR |
| Hendiani et al. 46 | Outpatients | ACR KOA criteria, >3 mo | Comorbidities, a history of trauma, prior surgery, use of recreational drugs (1 y) | QST | NeuP b | 28 (4 M, 24 F) | 53.2 ± 2.1 (33-70) | NR | 4.0 ± 0.6 (0.5-21) |
| Hochmann et al. 48 | Community | Knee pain, no TKA | Inflammatory arthritis, knee surgery | mPDQ | NeuP Prev d | 171 (39 M, 132 F) | 76 (67-99) | NR | NR |
| Hochmann et al. 47 | Outpatients + community | Symptomatic KOA x-ray confirmed OA | Chronic non-OA pain, comorbidities, a history of trauma or surgery | mPDQ, QST | NeuP b | 36 (57) (6 M, 30 F) | 60.7 ± 6.8 | 29.8 (19.5-62.3) | 10.1 (0.2-55.0) |
| Imamura et al. 49 | SurgeryList | Women, ACR KOA criteria, K-L2-4, VAS ≥4/10 | Other joints OA, comorbidities a | QST | NeuP b | 62 (0 M, 62 F) | 71.1 ± 6.6 | NR | 8.3 ± 6.3 |
| Jakorinne et al. 50 | Outpatients + community | Painful KOA, K-L1-4, age >18, Finnish speaking | Lesion at QST site, previous surgery, comorbidities, a analgesic | QST | NeuP b | 33 | (50-70) | NR | NR |
| Kavchak et al. 51 | Outpatients + community | K-L ≥2 | TKA, comorbidities a | QST | NeuP b | 16 (3 M, 13 F) | 52 ± 8 (39-61) | 38.3 ± 7.1 | 4.1 ± 4.0 |
| Kim et al. 52 | SurgeryList | Unilateral degenerative KOA | Inflammatory arthritis, ROM limitation >20°, history of knee surgery, comorbidities a | CSI | NeuP b | 161 (22 M, 139 F) | 71.2 ± 6.7 (53-85) | 25.6 ± 3.2 (16.0-38.2) | 7.5 ± 5.3 (0.5-25) |
| Kim et al. 26 | SurgeryList | KOA | ASA ≥III, drug or alcohol abuse, opioid (4 w), change in analgesic (2 w) obesity, spinal fusion, neurological disorders | CSI | NeuP b | 91 (0 M, 91 F) | 70.2 ± 5.7 | NR | NR |
| King et al. 53 | Community | Age 45-85, African American or non-Hispanic whites. ACR KOA criteria | Daily opioids use, major knee surgery, comorbidities a | QST | NeuP b | 209 (63 M, 146 F) | 57.3 (54.8-59.5) | 31.2 (27.9-35.1) | 3.3 (1.0-6.0) |
| Koh et al. 79 | SurgeryList | Patients undergone TKA for primary knee OA | Postoperative complications, ASA >2, centrally acting drugs, comorbidities a | CSI | NeuP b | 222 (21 M, 201 F) | 70 (57-83) | 26.4 (17.8-38.1) | NR |
| Kuni et al. 54 | SurgeryList | Patients undergoing TKA | Neurological and psychiatric diseases, infection of the joints, chronic low back pain | QST | NeuP b | 50 (23 M, 27 F) | 66 (44-77) | NR | 7 |
| Kurien et al. 55 | SurgeryList | Chronic KOA | Hip OA, comorbidities, a contraindication to MRI | PDQ, QST | NeuP b | 50 (20 M, 30 F) | 66.4 ± 8.3 | 30 (27-39) | 4.7 (2.9-7.2) |
| Lee et al. 56 | Outpatients | Painful KOA | Anxiety, pregnancy, infections, no neuropathy, no cardiovascular or AI disease | QST | NeuP b | 26 (6 M, 20 F) | 59.0 ± 7.5 | 27.5 (24.8-32.6) | NR |
| Lewis et al. 57 | SurgeryList | KOA pain >3 | Contraindications to MRI, neurological conditions, non-English speakers | QST | NeuP b | 29 (15 M, 14 F) | 68 ± 10 | 31 ± 5.7 | NR |
| Lluch Girbes et al. 58 | Outpatients | Chronic KOA pain (3 mo) | Previous joint replacement, comorbidities a | PDQ, QST | NeuP b | 53 (19 M, 34 F) | 70.2 ± 7.4 | 29.9 ± 3.9 | 7.5 ± 6 |
| Mesci et al. 59 | Outpatients | Age >40, knee pain, KOA according to ACR criteria | History of trauma or surgery, comorbidities a | PDQ, QST | NeuP b | 60 (14 M, 46 F) | 62.9 ± 10.5 | 29.6 ± 4.9 | 4.5 (1-20) |
| Moreton et al. 22 | Outpatients | Radiographic painful KOA (>3 mo) | Inflammatory rheumatic condition, knee surgery (3 mo), non-English speakers. | PDQ, S-LANSS, QST | NeuP b | 192 (90 M, 102 F) | 67 ± 10 | NR | 9 ± 9 |
| Moss et al. 62 | Community | KOA based on ACR | Comorbidities a | QST | NeuP b | 23 (10 M, 13 F) | 68.5 ± 8.5 (55-82) | 26.9 ± 4.5 | 8.26 (2-10) |
| Moss et al. 60 | Community | Painful KOA (VAS >3/10) | Comorbidities, a lower limb injury or surgery | PDQ, QST | Oth c | 80 (36 M, 44 F) | 65 (50-86) | 28.3 (20.3-38.3) | (2-30) |
| Moss et al. 61 | Community | KOA based on ACR criteria, pain >4/10 | Comorbidities, a lower limb injury or surgery | PDQ, QST | NeuP b | 130 (62 M, 68 F) | 66 (50-88) | NR | NR |
| Othori et al. 64 | Outpatients | Knee pain >1 mo, radiographic KOA | History of knee surgery, infection, and RA | PDQ | NeuP Prev d | 92 (21 M, 71 F) | 70.3 ± 8.0 (53-81) | NR | 3.1 ± 0.7 (0.1-15) |
| Othori et al. (2) 63 | Outpatients | Knee pain >1 mo, radiographic KOA | Previous knee surgery, infection, or RA | PDQ | Oth c | 89 (26 M, 63 F) | 70.0 ± 8.0 | NR | 3.0 ± 0.7 |
| Osgood, et al. 65 | Community | Age >21, painful KOA, pain 3/10 | NR | New index QST | NeuP b | 20 (6 M, 14 F) | 59.5 (30-83) | NR | 11.9 ± 7 |
| Oteo-Alvaro et al. 66 | Outpatients | Age ≥65 KOA (K-L 0-4) | Previous surgery, other source of pain | DN4 | NeuP Prev d | 2,167 (2,992) (762 M, 1,405 F) | 73.1 ± 5.4 | 28.7 ± 4.0 | 7.3 ± 5.5 |
| Petersen et al. 67 | SurgeryList | Severe KOA, scheduled for TKA | Fibromyalgia, RA, previous fractures | QST | NeuP | 135 (51 M, 84 F) | 67.6 ± 5.5 (44-89) | 30.2 ± 5.2 | NR |
| Phillips et al. 25 | SurgeryList | KOA | NR | PDQ | NeuP Prev d | 96 (42 M, 54 F) | 70.6 (48-89) | NR | NR |
| Polat et al. 68 | Outpatients | Painful KOA (>3 mo) | Previous surgery, infection, comorbidities, a medical treatment for NP | PDQ | NeuP Prev d | 109 (16 M, 93 F) | 62.5 ± 8.5 (44-81) | 33.7 ± 6.0 | 4.2 ± 3.5 |
| Power et al. 69 | SurgeryList | End-stage KOA, age >18 English speaking | TKA, posttraumatic or inflammatory forms of arthritis | PDQ | NeuP Prev d | 437 (184 M, 253 F) | NR | NR | NR |
| Pujol et al. 71 | Outpatients | KOA based on ACR criteria, >44 y, knee pain >3 mo, VAS pain 8-11 | Analgesic for 3 d, stable pain, steroids (3 mo) or HA (6 mo), antidepressants or anticonvulsive, comorbidities a | QST | NeuP b | 60 (17 M, 43 F) | 66.7 ± 7.8 | NR | NR |
| Radwan et al. 72 | Outpatients | KOA based on ACR criteria | Knee infection or surgery, comorbidities, a medical treatment for NP | DN4 | NeuP Prev d | 165 (319) (21 M, 144 F) | 53.1 | NR | 39.5 |
| Rakel et al. 73 | Outpatients/community | Age 18-95, KOA | Inability to walk, change in medication (3 w); VAS ≤2; knee surgery, knee infection (4 w), comorbidities a | QST | NeuP b | 75 (14 M, 61 F) | 56 ± 1.4 | 36.3 ± 0.9 | 6 ± (3-10) |
| Roubille et al. 74 | Outpatients | Age ≥40, primary KOA. according to ACR criteria, K-L 2-3, walk VAS ≥40 mm | Other bone or joint diseases, comorbidities restricting knee function, contraindication to MRI, steroids (12 w), HA (26 w) | PDQ | NeuP b | 50 (17 M, 33 F) | 64.2 ± 8.6 | 31.2 ± 5.1 | 7.8 ± 2.8 |
| Soni et al. 23 | SurgeryList | NR | NR | mPDQ, QST | NeuP b | 24 (13 M, 11 F) | 68.3 ± 8.9 | NR | 3.4 (1.5-9.0) |
| Tiendrebeogo et al. 80 | Outpatients | Age 40-85, KOA based on ACR criteria, VAS 3-8, non-respondent to NSAID or weak opioids | KOA with Flares, hip OA, rheumatic comorbidities, knee misalignment, steroids (3 mo) or HA (9 mo) | DN4 | Oth c | 187 (83 M, 104 F) | 64.7 (63.2-66.2) | 27.7 (27.0-28.4) | 46.5 (37.1-56.0) |
| Valdes et al. 76 | Outpatients | KOA (K-L >2), age ≥40 | No TKR or THA | PDQ | NeuP b | 139 (55 M, 84 F) | 63.9 ± 9.2 | 29.4 ± 4.81 | 10.7 ± 9.9 |
| Vas et al. 75 | NR | Pain, stiffness, function compromised in both knees | NR | PDQ | Oth c | 10 (20) (1 M, 9 F) | 61 ± 10.6 (49-82) | NR | NR |
| Wright, et al. 77 | NR | Painful KOA (VAS >3/10) | Comorbidities, a lower limb injury or surgery (<6 m) | QST | NeuP Prev d | 80 (36 M, 44 F) | 64 (50-86) | 38% obese | NR |
| Wylde et al. 78 | SurgeryList | KOA, being pain free in their right forearm | Cognitive impairment | QST | NeuP Prev d | 107 (56 M, 51 F) | 69 ± 8.5 | NR | 6 (3-10) |
M = males; F = females; SD = standard deviation; BMI = body mass index; KOA = knee osteoarthritis; TKA = total knee arthroplasty; MRI = magnetic resonance imaging; PDQ = PainDETECT questionnaire; ACR = American College of Rheumatology; QST = quantitative sensory testing; NR = not reported; ECG = electrocardiogram; K-L = Kellgren-Lawrence; d = days; w = weeks; DN4 = Douleur Neuropathic 4; y = years; mPDQ = modified PainDETECT questionnaire; mo = months; NRS = Numeric Rating Scale; S-LANSS = Leeds Assessment of Neuropathic Symptoms and Signs; VAS = Visual Analogue Scale; RA = rheumatoid arthritis; OA = osteoarthritis; CSI = central sensitization index; ASA = American Society of Anesthesiologists; pts = patients; Oth = Pain sensitization was documented only as baseline patient characteristic ; NeuP Prev = Evaluation of the prevalence of pain sensitization in knee OA as a primary aim; FKT = Physiotherapy; IA = intra-articular; HA = hyaluronic acid; ROM = range Of motion; NP = neurpathic pain; NSAID = non-steroidal antinflammaroty drugs; THA = total hip arthroplasty.
Major medical, rheumatologic, neurological, or psychiatric comorbidities that may influence pain perception.
Studies focused on relevant features of pain sensitization but not on its prevalence.
Pain sensitization was documented only as baseline patient characteristic.
Evaluation of the prevalence of pain sensitization in knee OA as a primary aim.
Pain Sensitization in Knee OA
The meta-analysis of proportion (Fig. 2) considering all the detection methods documented a prevalence of pain sensitization of 20% (95% confidence interval [CI] = 16%-26%), with a significant heterogeneity of results (I2 = 89%, P < 0.001). Sub-analyses were performed for the different detection methods used. PainDETECT was the most commonly used questionnaire and documented an overall prevalence of 17% (18% in the studies using the original PainDETECT and 11% in the studies using a modified form). Regarding the other questionnaires, the documented prevalence was 25% with DN4, 31% with S-LANSS, and 33% with CSI. Among the studies that used static QST protocols to evaluate pain sensitization, the documented prevalence was 29% using PPT, 27% using cold pain threshold (CPT), and 10% using heat pain threshold (HPT). In the studies evaluating pain response with dynamic QST protocols, a prevalence of 17% and 55% was reported in terms of abnormal TS and abnormal CPM, respectively.
Figure 2.
Prevalence of pain sensitization in the patients with knee osteoarthritis (forest plot). Both the fixed and random effect analyses are reported. The results of the random effect analysis are considered more reliable due to the documented heterogeneity in the included studies.
In the meta-analysis comparing pain sensitivity of knee OA patients and healthy controls (Fig. 3), a lower PPT was documented in the affected subjects, both in terms of local PPT (SMD = −1.00, 95% CI = −1.67 to −0.32, P = 0.007) and distant PPT (SMD = −0.54, 95% CI = −0.76 to −0.31, P < 0.001).
Figure 3.
Forest plot of the comparisons of local and distant pressure pain threshold in patients with knee osteoarthritis and healthy controls. Results are reported as standardized mean difference (SMD). The random effect model was used.
Factors Associated with Pain Sensitization
To evaluate the effect of study and patient characteristics on the prevalence of pain sensitization, a meta-regression analysis was performed. The diagnostic tool used (R2 = 41%, P = 0.01) and the aim of the study (R2 = 35%, P = 0.01) were associated with the documented prevalence. There was an association tendency also for pain duration (R2 = 21%, P = 0.07) and the category of the journal (R2 = 37%, P = 0.07). Number of patients included, publication year, country of the study, appropriateness of the inclusion criteria, recruitment technique, and patient characteristics such as sex, age, BMI, baseline pain intensity, and related symptoms were not significantly associated with the reported prevalence. A multiple meta-regression model was created with the identified associated factors (diagnostic tool, aim of the study, year, category of the journal). The only variable, whose significance was confirmed by multiple regression, was the diagnostic tool used, being the use of PainDETECT significantly associated with a lower documented prevalence (P = 0.03).
Quality of the Included Studies
The sample was considered representative in all included studies, except for the studies of Kim et al. and Imamura et al., which enrolled only women, and the study of Rabuille et al., which did not specify if the 50 patients included were recruited progressively or differentiated by the presence of neuropathic pain to create 2 groups with 25 patients each. The recruitment technique was appropriate in all but 4 studies, which did not specify how patients were recruited. Only 3 out of 46 studies had an adequate sample size (estimated as at least 246 patients; please refer to “Materials and Methods” section). A complete description of the study sample was reported in 24 studies. In 6 studies, there were missing data at follow-up, although it was not clear if this could influence the documented results. There is no standard measure to evaluate the presence of pain sensitization; thus, no study had the possibility to satisfy this criterium. All but 11 studies used a reliable measure to document the presence of pain sensitization: Only PainDETECT and QST protocols of the affected patients compared with those of healthy controls were considered reliable because the reliability of other self-reported questionnaires has never been confirmed by the literature.22,81 In 15 studies, the number of patients with pain sensitization was not available; thus, their statistical analysis was considered inappropriate. Most of the confounding factors were identified and accounted for in 36 studies, excluding patients with comorbidities that may influence the prevalence of pain sensitization. None of the studies evaluated the prevalence of pain sensitization in specific sub-populations.
Discussion
The main finding of this meta-analysis is that pain sensitization has a high prevalence in knee OA, representing a relevant component of symptoms suffered by many patients. Depending on the diagnostic tool used, the documented prevalence ranges from 10% in studies testing the decrease in HPTs to 56% in studies evaluating the reduction of CPM, underlining the differences based on the various tested pathways and, thus, the need to pursue exhaustive and standardized methods to evaluate pain sensitization.
Pain sensitization should not be overlooked when managing knee OA and its presence should always be considered to properly address this burdening condition. According to the results of this meta-analysis, up to one-fifth of patients with knee OA presents an altered pain processing mechanism that, if disregarded, may lead to treatment failure and patient dissatisfaction. Indeed, several studies have shown that the presence of a “neuropathic” component of pain in musculoskeletal diseases may lead to an increased risk of pain chronicity and to worse outcomes after treatment.82-85 Patients identified as “sensitized” probably represents a sub-group of knee OA patients with a specific pain phenotype that could take advantage of a targeted approach. 86 Treatments specifically addressing pain sensitization have been developed and tested to improve the results of traditional approaches to OA.70,87 In particular, duloxetine, a serotonin and norepinephrine reuptake inhibitor acting on the central nervous system as analgesic drug, showed initial promising results in treating a part of the patients with pain poorly controlled with traditional analgesic drugs.88,89 The fact that these treatments, developed with the intention of managing the neuropathic component of pain, have also an effect in tempering knee OA pain in some patients, is often considered a proof of the involvement of pain sensitization in OA symptoms. 90 However, it is still not clear if the advantages of these approaches rely on their overall good results as analgesic treatments or on their specific effectiveness in sensitized patients, as the improvement of the evidence in this field is hindered by the difficulties in detecting pain sensitization in musculoskeletal conditions.90,91
The diagnosis of pain sensitization, in the absence of a gold standard, is based on physical exam, questionnaires, and QST protocols. 92 Questionnaires evaluate the presence of signs and symptoms, such as dull pain, tingling, prickling, and widespread diffusion of pain with allodynia, that differ from those present in patients suffering from nociceptive musculoskeletal pain.93,94 QST protocols can be divided into static and dynamic tests. Static tests measure the pain threshold of patients related to a specific noxious stimuli, commonly pressure, heat, or cold. 95 Dynamic tests are aimed at quantifying the active response of the nervous system to pain: CPM is estimated computing the difference between pain threshold at rest and pain threshold during a peripheral noxious stimulation 96 ; TS is measured as the difference between patient-reported pain severity at first and last noxious stimuli of a series of supra-threshold stimuli with a constant intensity. 97 This pool of examinations evaluates most of the features of abnormal pain processing, and the combination of these tests has been included in comprehensive protocols aimed at investigating the presence of pain sensitization.98,99 In this light, the meta-analysis on the comparison between knee OA patients and healthy controls confirmed the presence of a hypersensitivity state in affected patients. Nonetheless, a great variability of reported prevalence of pain sensitization in association to the different diagnostic tools used was documented not only among different trials but also when different tests were used in the same study.35,38
The literature reports conflicting findings regarding the correlation between the results of the QST protocols and that of the most used questionnaires. Even though they are frequently used to evaluate the neurological involvement in knee OA pain, such questionnaires have been developed to evaluate the presence of neuropathic-like symptoms 93 or to suspect a neuropathy,100,101 whose presence in knee OA has never been confirmed. 19 Other authors also suggested that some of the questionnaires used to assess the presence of pain sensitization rely on questions that may reflect a broader definition of sensitivity which includes depression, anxiety, stress, and neuroticism. 102 Moreover, while for painDETECT a correlation with an increased pain sensitivity has been documented, this was never reported for DN4 and S-LANSS, and no correlation was found for CSI, whose results appear to be mainly influenced by the psychologic profile of the patients.47,81 This raises questions on the suitability of available diagnostic methods, as underlined by the meta-regression, which identified the diagnostic tool used as the greatest source of heterogeneity in the meta-analysis of prevalence. In this light, new attempts to identify a new subset of individual phenotypic traits that correlate with the presence of pain sensitization are ongoing, but new research confirming the relevance of the identified factors is needed. 103
The linear meta-regression also showed that the aim of the article resulted to be significantly associated with the reported prevalence: The highest prevalence was reported by studies aimed at evaluating features of pain sensitization that were not directly related to its prevalence, an intermediate value was reported by studies specifically focused on the prevalence of pain sensitization, whereas the lowest prevalence was documented by studies where pain sensitization was only one of the outcomes reported in a trial aimed at evaluating other aspects of knee OA. The highest prevalence documented in the first group of studies could be due to selection bias: Being the aim of these studies the evaluation of a feature of pain sensitization, it could be possible that patients suspected to have hypersensitivity were more easily recruited than other patients. On the contrary, the lack of attention and, probably, of experience in evaluating pain sensitization could be the cause of the lower prevalence documented in trials that were not focused on this aspect. The meta-regression also found a tendency toward association between the documented prevalence and the category of the journal with a higher prevalence for those on orthopedic and rehabilitation, followed by articles published in pain category, in rheumatology category, and in medicine category journals. A possible explanation could be the difference among patients visited by different practitioners: Patients with more severe knee OA pain have been suggested to be more likely to be seen by an orthopedic surgeon than by a rheumatologist or a general practitioner, and greater pain severity has been previously linked to higher prevalence of pain sensitization.33,104,105 Finally, the meta-regression found a tendency toward the association between symptoms’ duration and the prevalence of pain sensitization with an increase of the reported prevalence in the studies, including patients with a greater symptoms’ duration. Pain chronicity is considered a key factor for developing pain sensitization. 106 The constant noxious stimulation from the affected peripheral tissues is considered the trigger necessary to develop hypersensitivity, 107 and this could explain why the duration of pain was associated with a higher reported prevalence of pain sensitization. However, while these aspects are interesting insights for further investigation, the multiple meta-regression found that the only independent variable associated with the documented prevalence was the diagnostic tool used.
The meta-regression also evaluated patient-related characteristics that could explain the reported heterogeneity and identify patients that are more prone to develop pain sensitization. This could be extremely helpful to delineate a group of patients that should be tested with the available protocols that are otherwise considered too expensive and time-consuming to be used for all patients in the daily clinical practice. 108 Unfortunately, most of the patient-related characteristics evaluated with the meta-regression were not associated with the reported prevalence, thus hindering the possibility to identify a phenotype of patients with knee OA more prone to present pain sensitization. Patient characteristics, such as female sex and older age, or symptom characteristics, such as pain intensity, that are traditionally reported as distinctive of sensitized patients were not found to be associated with a higher prevalence of pain sensitization in knee OA.109,110 Moreover, some factors that may be related to the presence of pain sensitization, such as the degree of knee OA, could not be analyzed in the meta-regression due to the paucity of data available in the included studies.
The impossibility to perform a characterization of sensitized patients represents a limitation of the current literature that is reflected in the present meta-regression. Some of the included trials lacked data on patient characteristics that could have been useful to strengthen this evaluation. This is indicative of the unyielding need to improve the quality of the existing literature on the topic, as also underlined by the evaluation of the risk of bias of the included studies. Moreover, even though some of the sources of heterogeneity were identified and accounted for, there was still a high residual heterogeneity in the results of this meta-analysis. Besides this, it should be noted that the reliability of questionnaires and QST protocols in determining the presence of pain sensitization is still debated. This limited the precision of estimate of the exact prevalence of pain sensitization. Regarding the comparison of healthy subjects and knee OA patients, meta-analyses on thermal pain thresholds and dynamic QST could have been useful to provide more interesting insight on the topic. Unfortunately, the literature lacks studies providing data on this comparison and, with the number of studies available (3 on HPTs, 2 on CPTs, and none on dynamic QST), a meta-analysis could have led to misleading conclusions. Moreover, the recent literature reported changes in cerebrospinal fluid composition that may be helpful in characterizing knee OA patients with pain sensitization, but the evaluation of these issues was besides the focus of this meta-analysis that was focused on altered pain perception. 18 Finally, in the meta-analysis comparing PPT of affected patients and healthy controls, the use of healthy controls without knee OA or pain as comparators does not allow for clear understanding of whether structural pathology versus pain symptomatology (or both) are relevant for the development of pain sensitization.
Nonetheless, this meta-analysis was able to document that pain sensitization plays a key role in OA pain and should be the focus of more research efforts to further understand how to evaluate and address this component in the symptoms of patients affected by knee OA. Knee OA pain presents features that are consistent with a significant degree of pain sensitization. There is a high heterogeneity in the reported results, mainly based on the diagnostic tool used. The identification of the best methods to detect pain sensitization is warranted to correctly evaluate and manage symptoms of patients affected by knee OA.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: No ethical approval needed for the present study.
ORCID iDs: Davide Previtali  https://orcid.org/0000-0002-0284-4368
https://orcid.org/0000-0002-0284-4368
Gianluigi Capone  https://orcid.org/0000-0003-0575-703X
https://orcid.org/0000-0003-0575-703X
References
- 1. Montero A, Mulero J-F, Tornero C, Guitart J, Serrano M. Pain, disability and health-related quality of life in osteoarthritis-joint matters: an observational, multi-specialty trans-national follow-up study. Clin Rheumatol. 2016;35(9):2293-305. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen U-SDT, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? a systematic review. Ann Rheum Dis. 2011;70(1):60-7. [DOI] [PubMed] [Google Scholar]
- 4. Eberly L, Richter D, Comerci G, Ocksrider J, Mercer D, Mlady G, et al. Psychosocial and demographic factors influencing pain scores of patients with knee osteoarthritis. PLoS One. 2018;13(4):e0195075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cubukcu D, Sarsan A, Alkan H. Relationships between pain, function and radiographic findings in osteoarthritis of the knee: a cross-sectional study. Arthritis. 2012;2012:984060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P. et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fingleton C, Smart K, Moloney N, Fullen B, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(7):1043-56. [DOI] [PubMed] [Google Scholar]
- 8. Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573-81. [DOI] [PubMed] [Google Scholar]
- 9. Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65(2):363-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74(4):682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Staud R. Is it all central sensitization? role of peripheral tissue nociception in chronic musculoskeletal pain. Curr Rheumatol Rep. 2010;12(6):448-54. [DOI] [PubMed] [Google Scholar]
- 12. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61(9):1226-34. [DOI] [PubMed] [Google Scholar]
- 14. Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-51. [DOI] [PubMed] [Google Scholar]
- 15. Smart KM, Blake C, Staines A, Doody C. Clinical indicators of “nociceptive,” “peripheral neuropathic” and “central” mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man Ther. 2010;15(1):80-7. [DOI] [PubMed] [Google Scholar]
- 16. Rowbotham MC. Is fibromyalgia a neuropathic pain syndrome? J Rheumatol Suppl. 2005;75:38-40. [PubMed] [Google Scholar]
- 17. Rasmussen PV, Sindrup SH, Jensen TS, Bach FW. Symptoms and signs in patients with suspected neuropathic pain. Pain. 2004;110(1-2):461-9. [DOI] [PubMed] [Google Scholar]
- 18. Bjurström MF, Blennow K, Zetterberg H, Bodelsson M, Waldén M, Dietz N, et al. Central nervous system monoaminergic activity in hip osteoarthritis patients with disabling pain: associations with pain severity and central sensitization. Pain Rep. 2022;7(1):e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Treede R-D, Jensen TS, Campbell JN, Cruccu G, Dostrovsky J, Griffin J, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630-5. [DOI] [PubMed] [Google Scholar]
- 20. Freynhagen R, Parada HA, Calderon-Ospina CA, Chen J, Rakhmawati Emril D, Fernandez-Villacorta FJ, et al. Current understanding of the mixed pain concept: a brief narrative review. Curr Med Res Opin. 2019;35(6):1011-8. [DOI] [PubMed] [Google Scholar]
- 21. IASP Terminology. Available from: https://www.iasp-pain.org/terminology?navItemNumber=576.
- 22. Moreton BJ, Tew V, das Nair R, Wheeler M, Walsh DA, Lincoln NB. Pain phenotype in patients with knee osteoarthritis: classification and measurement properties of painDETECT and self-report Leeds Assessment of Neuropathic Symptoms and Signs scale in a cross-sectional study. Arthritis Care Res. 2015;67(4):519-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soni A, Wanigasekera V, Mezue M, Cooper C, Javaid MK, Price AJ, et al. Central sensitization in knee osteoarthritis: relating presurgical brainstem neuroimaging and pain DETECT-based patient stratification to arthroplasty outcome. Arthritis Rheumatol. 2019;71(4):550-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. French HP, Smart KM, Doyle F. Prevalence of neuropathic pain in knee or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2017;47(1):1-8. [DOI] [PubMed] [Google Scholar]
- 25. Phillips J, Hopwood B, Arthur C, Stroud R, Toms A. The natural history of pain and neuropathic pain after knee replacement: a prospective cohort study of the point prevalence of pain and neuropathic pain to a minimum three-year follow-up. Bone Joint J. 2014;96-B(9):1227-33. [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Yoon KB, Yoon DM, Yoo JH, Ahn KR. Influence of centrally mediated symptoms on postoperative pain in osteoarthritis patients undergoing total knee arthroplasty: a prospective observational evaluation. Pain Pract. 2015;15(6):E46-53. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9. [DOI] [PubMed] [Google Scholar]
- 28. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofacial Sci. 2006;1:9-14. [Google Scholar]
- 30. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-8. [DOI] [PubMed] [Google Scholar]
- 31. Alshuft HM, Condon LA, Dineen RA, Auer DP. Cerebral cortical thickness in chronic pain due to knee osteoarthritis: the effect of pain duration and pain sensitization. PLoS One. 2016;11(9):e0161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arendt-Nielsen L, Egsgaard LL, Petersen KK. Evidence for a central mode of action for etoricoxib (COX-2 inhibitor) in patients with painful knee osteoarthritis. Pain. 2016;157(8):1634-44. [DOI] [PubMed] [Google Scholar]
- 33. Arendt-Nielsen L, Egsgaard LL, Petersen KK, Eskehave TN, Graven-Nielsen T, Hoeck HC, et al. A mechanism-based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur J Pain. 2015;19(10):1406-17. [DOI] [PubMed] [Google Scholar]
- 34. Arendt-Nielsen L, Jiang G, DeGryse R, Turkel C. Intra-articular onabotulinumtoxinA in osteoarthritis knee pain: effect on human mechanistic pain biomarkers and clinical pain. Scand J Rheumatol. 2017;46(4):303-16. [DOI] [PubMed] [Google Scholar]
- 35. Aşkın A, Özkan A, Tosun A, Demirdal ÜS, İsnaç F. Quality of life and functional capacity are adversely affected in osteoarthritis patients with neuropathic pain. Kaohsiung J Med Sci. 2017;33(3):152-8. [DOI] [PubMed] [Google Scholar]
- 36. Blikman T, Rienstra W, van Raay JJAM, Dijkstra B, Bulstra SK, Stevens M, et al. Neuropathic-like symptoms and the association with joint-specific function and quality of life in patients with hip and knee osteoarthritis. PLoS One. 2018;13(6):e0199165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bossmann T, Brauner T, Horstmann T. Differences in pain intensity in anti-and pro-nociceptive pain profile subgroups in patients with knee osteoarthritis. Pain Manag. 2018;8(1):27-36. [DOI] [PubMed] [Google Scholar]
- 38. Cardoso JS, Riley JL, 3rd, Glover T, Sibille KT, Bartley EJ, Goodin BR, et al. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain. 2016;157(9):2104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Courtney CA, Steffen AD, Fernández-De-Las-Peñas C, Kim J, Chmell SJ. Joint mobilization enhances mechanisms of conditioned pain modulation in individuals with osteoarthritis of the knee. J Orthop Sports Phys Ther. 2016;46(3):168-76. [DOI] [PubMed] [Google Scholar]
- 40. Dua AB, Neogi T, Mikolaitis RA, Block JA, Shakoor N. Somatosensation in OA: exploring the relationships of pain sensitization, vibratory perception and spontaneous pain. BMC Musculoskelet Disord. 2018;19(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fingleton C, Smart KM, Doody CM. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain. 2017;33(5):395-404. [DOI] [PubMed] [Google Scholar]
- 42. Fitzsimmons M, Carr E, Woodhouse L, Bostick GP. Development and persistence of suspected neuropathic pain after total knee arthroplasty in individuals with osteoarthritis. PM R. 2018;10(9):903-9. [DOI] [PubMed] [Google Scholar]
- 43. Garip Y, Eser F, Kılıçarslan A, Bodur H. Prevalence of neuropathic pain in rheumatic disorders: association with disease activity, functional status and quality of life. Arch Rheumatol. 2015;30:231-7. [Google Scholar]
- 44. Golob M, Marković I, Zovko N, Šakić D, Gudelj-Gračanin A, Morović-Vergles J. Do we pay enough attention to neuropathic pain in knee osteoarthritis patients? Acta Clin Croat. 2018;57(1):16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64(9):2907-16. [DOI] [PubMed] [Google Scholar]
- 46. Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain. 2003;4(4):203-11. [DOI] [PubMed] [Google Scholar]
- 47. Hochman J, Davis A, Elkayam J, Gagliese L, Hawker G. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1236-42. [DOI] [PubMed] [Google Scholar]
- 48. Hochman J, Gagliese L, Davis A, Hawker G. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19(6):647-54. [DOI] [PubMed] [Google Scholar]
- 49. Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, De Souza LPM, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Care Res. 2008;59(10):1424-31. [DOI] [PubMed] [Google Scholar]
- 50. Jakorinne P, Haanpää M, Arokoski J. Reliability of pressure pain, vibration detection, and tactile detection threshold measurements in lower extremities in subjects with knee osteoarthritis and healthy controls. Scand J Rheumatol. 2018;47(6):491-500. [DOI] [PubMed] [Google Scholar]
- 51. Kavchak AJE, Fernández-De-Las-Peñas C, Rubin LH, Arendt-Nielsen L, Chmell SJ, Durr RK, et al. Association between altered somatosensation, pain, and knee stability in patients with severe knee osteoarthrosis. Clin J Pain. 2012;28(7):589-94. [DOI] [PubMed] [Google Scholar]
- 52. Kim MS, Koh IJ, Lee SY, In Y. Central sensitization is a risk factor for wound complications after primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3419-28. [DOI] [PubMed] [Google Scholar]
- 53. King CD, Sibille KT, Goodin BR, Cruz-Almeida Y, Glover TL, Bartley E, et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1243-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuni B, Wang H, Rickert M, Ewerbeck V, Schiltenwolf M. Pain threshold correlates with functional scores in osteoarthritis patients. Acta Orthop. 2015;86(2):215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kurien T, Arendt-Nielsen L, Petersen KK, Graven-Nielsen T, Scammell BE. Preoperative neuropathic pain-like symptoms and central pain mechanisms in knee osteoarthritis predicts poor outcome 6 months after total knee replacement surgery. J Pain. 2018;19(11):1329-41. [DOI] [PubMed] [Google Scholar]
- 56. Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res. 2011;63(3):320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lewis GN, Parker RS, Sharma S, Rice DA, McNair PJ. Structural brain alterations before and after total knee arthroplasty: a longitudinal assessment. Pain Medicine. 2018;19(11):2166-76. [DOI] [PubMed] [Google Scholar]
- 58. LluchGirbes E, Duenas L, Barbero M, Falla D, Baert IA, Meeus M, et al. Expanded distribution of pain as a sign of central sensitization in individuals with symptomatic knee osteoarthritis. Phys Ther. 2016;96(8):1196-207. [DOI] [PubMed] [Google Scholar]
- 59. Mesci N, Mesci E, Kulcu DG. Association of neuropathic pain with ultrasonographic measurements of femoral cartilage thickness and clinical parameters in patients with knee osteoarthritis. J Phys Ther Sci. 2016;28(8):2190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moss P, Benson HAE, Will R, Wright A. Fourteen days of etoricoxib 60 mg improves pain, hyperalgesia and physical function in individuals with knee osteoarthritis: a randomized controlled trial. Osteoarthritis Cartilage. 2017;25(11):1781-91. [DOI] [PubMed] [Google Scholar]
- 61. Moss P, Benson HAE, Will R, Wright A. Patients with knee osteoarthritis who score highly on the PainDETECT Questionnaire present with multimodality hyperalgesia, increased pain, and impaired physical function. Clin J Pain. 2018;34(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moss P, Knight E, Wright A. Subjects with knee osteoarthritis exhibit widespread hyperalgesia to pressure and cold. PLoS One. 2016;11(1):e0147526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ohtori S, Inoue G, Orita S, Takaso M, Eguchi Y, Ochiai N. et al. Efficacy of combination of meloxicam and pregabalin for pain in knee osteoarthritis. Yonsei Med J. 2013;54(5):1253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohtori S, Orita S, Yamashita M, Ishikawa T, Ito T, Shigemura T. et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med J. 2012;53(4):801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Osgood E, Trudeau JJ, Eaton TA, Jensen MP, Gammaitoni A, Simon LS. et al. Development of a bedside pain assessment kit for the classification of patients with osteoarthritis. Rheumatol Int. 2015;35(6):1005-13. [DOI] [PubMed] [Google Scholar]
- 66. Oteo-Alvaro A, Ruiz-Iban MA, Miguens X, Stern A, Villoria J, Sanchez-Magro I. High prevalence of neuropathic pain features in patients with knee osteoarthritis: a cross-sectional study. Pain Pract. 2015;15(7):618-26. [DOI] [PubMed] [Google Scholar]
- 67. Petersen KK, Arendt-Nielsen L, Finocchietti S, Hirata RP, Simonsen O, Laursen MB, et al. Age interactions on pain sensitization in patients with severe knee osteoarthritis and controls. Clin J Pain. 2017;33(12):1081-7. [DOI] [PubMed] [Google Scholar]
- 68. Polat CS, Dogan A, Sezgin Ozcan D, Koseoglu BF, Kocer Akselim S. Is there a possible neuropathic pain component in knee osteoarthritis? Arch Rheumatol. 2017;32(4):333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Power JD, Perruccio AV, Gandhi R, Veillette C, Davey JR, Syed K, et al. Neuropathic pain in end-stage hip and knee osteoarthritis: differential associations with patient-reported pain at rest and pain on activity. Osteoarthritis Cartilage. 2018;26(3):363-9. [DOI] [PubMed] [Google Scholar]
- 70. Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253-60. [DOI] [PubMed] [Google Scholar]
- 71. Pujol J, Martinez-Vilavella G, Llorente-Onaindia J, Harrison BJ, Lopez-Sola M, Lopez-Ruiz M, et al. Brain imaging of pain sensitization in patients with knee osteoarthritis. Pain. 2017;158(9):1831-8. [DOI] [PubMed] [Google Scholar]
- 72. Radwan A, Borai A. Neuropathic pain in Egyptian patients with primary knee osteoarthritis: relationship with functional status and radiological severity. Egypt Rheumatol. 2019;41(4):261-4. [Google Scholar]
- 73. Rakel B, Vance C, Zimmerman MB, Petsas-Blodgett N, Amendola A, Sluka KA. Mechanical hyperalgesia and reduced quality of life occur in people with mild knee osteoarthritis pain. Clin J Pain. 2015;31(4):315-22. [DOI] [PubMed] [Google Scholar]
- 74. Roubille C, Raynauld JP, Abram F, Paiement P, Dorais M, Delorme P, et al. The presence of meniscal lesions is a strong predictor of neuropathic pain in symptomatic knee osteoarthritis: a cross-sectional pilot study. Arthritis Res Ther. 2014;16(6):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vas L, Pai R, Khandagale N, Pattnaik M. Pulsed radiofrequency of the composite nerve supply to the knee joint as a new technique for relieving osteoarthritic pain: a preliminary report. Pain Physician. 2014;17(6):493-506. [PubMed] [Google Scholar]
- 76. Valdes AM, Suokas AK, Doherty SA, Jenkins W, Doherty M. History of knee surgery is associated with higher prevalence of neuropathic pain-like symptoms in patients with severe osteoarthritis of the knee. Semin Arthritis Rheum. 2014;43(5):588-92. [DOI] [PubMed] [Google Scholar]
- 77. Wright A, Benson HAE, Will R, Moss P. Cold pain threshold identifies a subgroup of individuals with knee osteoarthritis that present with multimodality hyperalgesia and elevated pain levels. Clin J Pain. 2017;33(9):793-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology. 2012;51(3):535-43. [DOI] [PubMed] [Google Scholar]
- 79. Koh IJ, Kang BM, Kim MS, Choi KY, Sohn S, In Y. How does preoperative central sensitization affect quality of life following total knee arthroplasty? J Arthroplasty. 2020;35(8):2044-9. [DOI] [PubMed] [Google Scholar]
- 80. Tiendrebeogo E, Choueiri M, Chevalier X, Conrozier T, Eymard F. Does the presence of neuropathic pain influence the response to hyaluronic acid in patients with knee osteoarthritis? Cartilage. 2021;13:1548S-56S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gervais-Hupé J, Pollice J, Sadi J, Carlesso LC. Validity of the central sensitization inventory with measures of sensitization in people with knee osteoarthritis. Clin Rheumatol. 2018;37(11):3125-32. [DOI] [PubMed] [Google Scholar]
- 82. Shaygan M, Böger A, Kröner-Herwig B. Clinical features of chronic pain with neuropathic characteristics: a symptom-based assessment using the Pain DETECT Questionnaire. Eur J Pain. 2013;17(10):1529-38. [DOI] [PubMed] [Google Scholar]
- 83. Lundblad H, Kreicbergs A, Jansson K-Å. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br. 2008;90(2):166-71. [DOI] [PubMed] [Google Scholar]
- 84. Skou ST, Graven-Nielsen T, Rasmussen S, Simonsen OH, Laursen MB, Arendt-Nielsen L. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain. 2013;154(9):1588-94. [DOI] [PubMed] [Google Scholar]
- 85. O’Leary H, Smart KM, Moloney NA, Blake C, Doody CM. Pain sensitization associated with nonresponse after physiotherapy in people with knee osteoarthritis. Pain. 2018;159(9):1877-86. [DOI] [PubMed] [Google Scholar]
- 86. Dell’Isola A, Allan R, Smith SL, Marreiros SSP, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. 2016;17(1):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hochberg MC, Wohlreich M, Gaynor P, Hanna S, Risser R. Clinically relevant outcomes based on analysis of pooled data from 2 trials of duloxetine in patients with knee osteoarthritis. J Rheumatol. 2012;39(2):352-8. [DOI] [PubMed] [Google Scholar]
- 88. Brown JP, Boulay LJ. Clinical experience with duloxetine in the management of chronic musculoskeletal pain. A focus on osteoarthritis of the knee. Ther Adv Musculoskelet Dis. 2013;5(6):291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Frakes E, Risser R, Ball T, Hochberg M, Wohlreich M. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27(12):2361-72. [DOI] [PubMed] [Google Scholar]
- 90. Lluch Girbés E, Nijs J, Torres-Cueco R, López Cubas C. Pain treatment for patients with osteoarthritis and central sensitization. Phys Ther. 2013;93(6):842-51. [DOI] [PubMed] [Google Scholar]
- 91. Allen K. Central pain contributions in osteoarthritis: next steps for improving recognition and treatment? Arthritis Res Ther. 2011;13(6):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D. et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14-27. [DOI] [PubMed] [Google Scholar]
- 93. Freynhagen R, Baron R, Gockel U, Tölle TR. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911-20. [DOI] [PubMed] [Google Scholar]
- 94. Santana JC, Santos VS, Gurgel RQ, Santana JC, Reis FP, Cuevas LE, et al. Agreement between the Douleur Neuropathique in 4 questions and Leeds Assessment of Neuropathic Symptoms and Signs questionnaires to classify neuropathic pain among patients with leprosy. Am J Trop Med Hyg. 2016;95(4):756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Uddin Z, MacDermid JC. Quantitative sensory testing in chronic musculoskeletal pain. Pain Med. 2016;17(9):1694-703. [DOI] [PubMed] [Google Scholar]
- 96. Yarnitsky D, Bouhassira D, Drewes A, Fillingim R, Granot M, Hansson P, et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. 2015;19(6):805-6. [DOI] [PubMed] [Google Scholar]
- 97. Nie H, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal summation of pain evoked by mechanical pressure stimulation. Eur J Pain. 2009;13(6):592-9. [DOI] [PubMed] [Google Scholar]
- 98. Rolke R, Baron R, Maier C, Tölle TR, Treede R-D, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS):standardized protocol and reference values. Pain. 2006;123(3):231-43. [DOI] [PubMed] [Google Scholar]
- 99. Geber C, Klein T, Azad S, Birklein F, Gierthmühlen J, Huge V. et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS):a multi-centre study. Pain. 2011;152(3):548-56. [DOI] [PubMed] [Google Scholar]
- 100. Bennett M. The LANSS Pain Scale: the Leeds Assessment of Neuropathic Symptoms and Signs. Pain. 2001;92(1-2):147-57. [DOI] [PubMed] [Google Scholar]
- 101. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1-2):29-36. [DOI] [PubMed] [Google Scholar]
- 102. Adams GR, Gandhi W, Harrison R, van Reekum CM, Gilron I, Salomons TV. Do “central sensitization” questionnaires reflect measures of nociceptive sensitization or psychological constructs? protocol for a systematic review. Pain Rep. 2021;6(4):e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Akin-Akinyosoye K, Frowd N, Marshall L, Stocks J, Fernandes GS, Valdes A, et al. Traits associated with central pain augmentation in the Knee Pain in the Community (KPIC) cohort. Pain. 2018;159(6):1035-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Katz JN, Solomon DH, Schaffer JL, Horsky J, Burdick E, Bates DW. Outcomes of care and resource utilization among patients with knee or shoulder disorders treated by general internists, rheumatologists, or orthopedic surgeons. Am J Med. 2000;108(1):28-35. [DOI] [PubMed] [Google Scholar]
- 105. Mazzuca SA, Brandt KD, Katz BP, Dittus RS, Freund DA, Lubitz R, et al. Comparison of general internists, family physicians, and rheumatologists managing patients with symptoms of osteoarthritis of the knee. Arthritis Care Res. 1997;10(5):289-99. [DOI] [PubMed] [Google Scholar]
- 106. Arendt-Nielsen L, Graven-Nielsen T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Curr Pain Headache Rep. 2003;7(5):355-61. [DOI] [PubMed] [Google Scholar]
- 107. Martindale J, Wilson A, Reeve A, Chessell I, Headley P. Chronic secondary hypersensitivity of dorsal horn neurones following inflammation of the knee joint. Pain. 2007;133(1-3):79-86. [DOI] [PubMed] [Google Scholar]
- 108. Birklein F, Sommer C. Pain: quantitative sensory testing—a tool for daily practice? Nat Rev Neurol. 2013;9(9):490-2. [DOI] [PubMed] [Google Scholar]
- 109. Bartley EJ, King CD, Sibille KT, Cruz-Almeida Y, Riley JL, 3rd, Glover TL, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res. 2016;68(4):472-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Riley JL, 3rd, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15(3):272-82. [DOI] [PMC free article] [PubMed] [Google Scholar]