Abstract
Objective
To investigate the expression of Hedgehog (HH) signaling pathway proteins in knee articular cartilage from Kashin-Beck disease (KBD) and osteoarthritis (OA) patients.
Methods
Knee articular cartilage samples were collected from normal (N), OA, and KBD adults (aged 38-60 years) and divided into 3 groups with 6 subjects in each group. The localization of the HH pathway proteins bone morphogenetic protein 2 (BMP2), bone morphogenetic protein 4 (BMP4), Sonic hedgehog (SHH), and Indian hedgehog (IHH) was observed with the microscope after immunohistochemical (IHC) staining. Positive staining cell rates of each proteins were compared.
Results
The strongest stainings of all proteins were observed in the middle zones of all 3 groups. The positive staining rates of BMP4 and IHH were significantly lower in the OA and KBD groups than those in the N group in all 3 zones. The positive staining rates of BMP2 and SHH tend to be lower in the OA and KBD groups than those in the N group in the deep zone, while higher in the OA and KBD groups than those in the N group in superficial and middle zones.
Conclusions
Altered expression of the HH pathway proteins BMP2, BMP4, SHH, and IHH was found in OA and KBD articular cartilage. There seemed to be a compensatory effect between SHH and IHH in cartilage damage. Further studies on the pathogenesis of OA and KBD may be carried out from these aspects in the future.
Keywords: Kashin-Beck disease, osteoarthritis, Hedgehog pathway, articular cartilage, immunohistochemistry
Introduction
Osteoarthritis (OA) is one of the most common articular inflammatory diseases, characterized by the destruction and degradation of articular cartilage, which often causes joint morphological changes, chronic joint pain, and disability. 1 Kashin-Beck disease (KBD) is a kind of endemic and chronic osteoarthropathy that leads to OA in its advanced stage. In contrast to OA, the main pathological manifestations of KBD include chondrocytes necrosis in deep zone, degenerative changes of hyaline cartilage, and degradation of cartilage matrix.2,3 Due to the avascular properties of the cartilage, articular cartilage has a poor ability to regenerate and repair tissue after injury or degenerative disease, and the healing is always limited, which results in joint dysfunction.4,5 All these impair the work productivity and quality of life of the patients, imposing a serious burden on families and society.6 -8
It is known that articular cartilage is a layer of connective tissue covering the articulating bone ends. Its main function is to conduct and distribute loads, maintain and withstand mechanical stress, and absorb stress generated by joint loading.9,10 Chondrocytes are the only living cells located in the cartilage; they produce an extracellular matrix and maintain the functional balance between extracellular matrix degradation and repair. 11 It has recently been confirmed that the Hedgehog (HH) signaling pathway plays an important role in the development of chondrogenesis and OA.12 -14 The growth and development of chondrocytes require the HH signaling pathways, 15 which also function as morphogens and differentiation guiding factors.
The protein components of the HH pathway include bone morphogenetic protein 2 (BMP2), bone morphogenetic protein 4 (BMP4), Sonic hedgehog (SHH), Indian hedgehog (IHH), and so on. IHH increases cell proliferation and cartilage formation by converting mechanical strain to proliferative signals. 16 A previous study found that IHH expression correlated with OA progress, induced chondrocyte hypertrophy, and increased type X collagen (COL10) and matrix metalloproteinase (MMP) expression. 17 SHH signaling is required for the initiation of vertebral chondrogenesis. The cells of mice lacking SHH failed to produce vertebrae. SHH can cooperate with BMP signaling to promote cartilage formation. 18 Bone morphogenetic proteins (BMPs) belong to the family of cytokines associated with bone development and are produced in mesenchymal cells, osteoblasts, and chondrocytes. BMPs can induce cartilage growth at different sites in the body and stimulate ectomesenchymal cells to differentiate into cartilage cells. 19 HH proteins interact with BMPs coordinating chondrocyte proliferation and differentiation. 20 Both BMPs and HH proteins are required to maintain normal proliferation. BMP signaling delays the process of hypertrophic differentiation itself. They may also play an important role in cartilage damage and disease. 21
The HH pathway plays an important role in the performance of physiological functions in cartilage tissue. Cartilage damage may be associated with abnormal or impaired expression of the HH signaling pathway proteins. Previous studies have not focused on the expression and potential pathogenesis of the HH pathway in KBD cartilage damage. This study focused on the relationship between key proteins in the HH signaling pathway and cartilage damage in KBD and OA to provide new clues for the exploration of their pathogenesis.
Materials and Methods
Cartilage Sample Collection
This study was permitted by the Human Ethics Committee of Xi’an Jiaotong University (No. 2017-196); all procedures performed in this study involving human participants were following the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the cartilage sample donors provided signed informed consent for the study participation and publication of their individual clinical details and images before the study started. In all, 18 knee articular cartilage samples were collected. Six KBD samples were obtained from KBD patients living in Linyou, a KBD-endemic area of Shaanxi province. Six OA samples were obtained from OA patients living in Xi’an, a non-KBD-endemic area of Shaanxi province. Six normal (N) samples were collected from donors in non-KBD endemic regions, who suffered from traffic accidents and had undergone knee replacement surgery. Detailed characteristics of subjects are shown in Table 1 . KBD was diagnosed based on KBD clinical diagnosis criteria (WST 207-2010) in China. 22 OA was diagnosed based on the OA Index of Western Ontario and McMaster Universities. 23
Table 1.
Characteristics of Experiment Subjects.
| N | OA | KBD | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Age | Sex | Sample | Age | Sex | Sample | Age | Sex |
| N1 | 45 | F | OA1 | 54 | F | KBD1 | 50 | F |
| N2 | 38 | F | OA2 | 48 | F | KBD2 | 44 | F |
| N3 | 48 | M | OA3 | 45 | M | KBD3 | 50 | M |
| N4 | 45 | M | OA4 | 60 | F | KBD4 | 58 | F |
| N5 | 55 | M | OA5 | 60 | M | KBD5 | 57 | M |
| N6 | 54 | M | OA6 | 58 | M | KBD6 | 55 | M |
| Mean | 47.50 | — | Mean | 54.17 | — | Mean | 52.33 | — |
, FAge = 2.485, both P values >0.05.
N = normal adult; OA = osteoarthritis; KBD = Kashin-Beck disease; F = female; M = male.
The cartilage tissues were collected within 8 hours after surgery and fixed in 4% paraformaldehyde. The same weight of knee cartilage tissue was taken from the same part with a scalpel. (Sampling site: full-thickness cartilage blocks with an area of about 4 mm × 4 mm were cut from the loading area in front of the medial and lateral sides of the tibial plateau and the loading area in front of the medial.) After fixing in 4% (w/v) paraformaldehyde for 2 to 3 days and decalcifying the samples in 10% (w/v) ethylenediaminetetraacetic acid (EDTA) for 4 weeks, the samples were embedded in paraffin. Slices about 5 μm were cut from each cartilage tissue wax.
Immunohistochemical (IHC) Staining
The IHC staining of N, OA, and KBD samples was performed at the same time. All slices were first laid out in a water bath with the water temperature of about 40 °C and then pasted on a glass slide. The slices were then placed in a baking machine at 60 °C for 3 hours, and they were then deparaffinized and rehydrated in a series of gradually decreasing concentrations of ethanol. The activity of endogenous peroxidase was quenched by using 0.3% (w/v) hydrogen peroxide (H2O2) for 10 minutes at room temperature, followed by incubation with 10 M urea for 20 min and then with 2 mg/mL hyaluronidase (Sigma Aldrich, St. Louis, MO, USA) for 20 min. The nonspecific binding was blocked with 10% (v/v) normal goat serum for 15 minutes. Tissue sections were incubated with primary antibodies: BMP2 (catalog no.18933-1-AP; Proteintech Group, USA), BMP4 (catalog no.12492-1-AP; Proteintech Group), SHH (catalog no. ab53281; Abcam, UK), and IHH (catalog no. ab52919; Abcam, UK) in 1% bovine serum albumin (1:50 dilution in phosphate-buffered saline [PBS]) at 4 °C overnight. For the negative controls, the primary antibodies were replaced with PBS containing rabbit IgG. After washing with PBS, the sections were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:500) at 37 °C for 30 minutes. After extensive washing, the sections were visualized using a diaminobenzidine (DAB) kit (ZLI-9017; ZSGB-BIO, China) according to the manufacturer’s protocols and counterstained with hematoxylin. The negative controls (negative control rabbit IgG) showed no nonspecific staining.
Image Capture and Analysis
Representative regions were selected and photographed using an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan) equipped with digital image acquisition. When viewed at 100x magnification, all images have a resolution of 1,200 × 1,600. Images were stored in TIFF format. Each cartilage section was manually divided into 3 parts: superficial, middle, and deep zones. The discrepancy of cellular morphology, density, and arrangement, as well as aggregate rate, was used to distinguish the superficial, middle, and deep zones. The long axis of superficial zone chondrocytes is parallel to the cartilage surface, and cells are small and relatively flat. Middle zone chondrocytes are larger, showing a round cell outline, and are randomly distributed in the cartilage matrix. Deep zone chondrocytes are arranged in a columnar manner perpendicular to the surface, and the cell size gradually increases. Six randomly selected areas in superficial, middle, and deep zones were captured from each sample, respectively.
The number of positively stained chondrocytes were counted using Image J cell counting software (NIH, USA). All the chondrocytes and the positively stained cells in the whole cartilage depth were counted in each image:
Statistical Analysis
The SPSS 22.0 software (SPSS Inc, USA) was used for data entry and analysis. As the data from all groups did not pass the normality test and homogeneity of variance test, the data were calculated as median (P25, P75). Kruskal-Wallis rank-sum test was used for comparisons. P < 0.05 was considered to indicate a statistically significant difference.
Results
The IHC staining showed that BMP2, BMP4, SHH, and IHH were positively stained in brownish-yellow color, and they were mainly localized in the chondrocytes. And the strongest BMP2, BMP4, SHH, and IHH stainings were observed in the middle zone of all 3 groups.
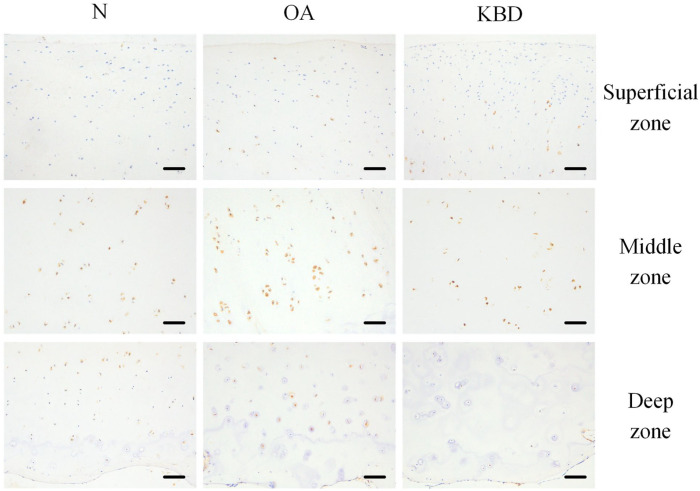
For BMP2, the strongest staining was shown in the middle zone of the OA and N groups, followed by the deep zone and then the superficial zone. But in the KBD group, the strongest staining was shown in the middle zone, followed by the superficial zone and then the deep zone. The positive staining rate of BMP2 in the deep zone was significantly lower in the KBD group than those in the OA and N groups ( Fig. 1 ; Table 2 ).
Figure 1.
Representative IHC staining of BMP2 in superficial, middle, and deep zones of articular cartilage in normal adults, OA and KBD patients. Scale bar 20 μm. IHC = immunohistochemistry; BMP2 = bone morphogenetic protein 2; OA = osteoarthritis; KBD = Kashin-Beck disease.
Table 2.
Positive Staining Cell Rates of BMP2, BMP4, SHH, and IHH and Their Comparisons in 3 Different Zones of Normal (N), OA, and KBD Cartilage (%).
| Enzyme | Zone | Group (positive staining cell rates, %) |
P values for multiple comparisons between groups after adjustment |
||||
|---|---|---|---|---|---|---|---|
| N | OA | KBD | N-OA | N-KBD | OA-KBD | ||
| BMP2 | Superficial | 9.39 (0.00, 56.70) | 12.36 (6.93, 32.45) | 22.26 (6.72, 40.40) | - | - | - |
| Middle | 80.15 (60.84, 92.64) | 85.41 (73.06, 96.37) | 82.12 (71.48, 93.08) | - | - | - | |
| Deep | 36.56 (15.62, 63.73) | 22.14 (14.03, 45.00) | 8.00 (3.24, 13.82) | 0.151 | <0.01** | <0.01** | |
| BMP4 | Superficial | 56.78 (35.79, 93.79) | 2.44 (0.00, 21.62) | 12.47 (1.53, 87.44) | <0.01** | 0.013* | 0.01* |
| Middle | 82.63 (27.12, 89.85) | 50.46 (7.00, 89.02) | 79.45 (67.83, 85.25) | - | - | - | |
| Deep | 20.22 (14.08, 32.13) | 2.04 (0.00, 33.45) | 7.39 (0.00, 32.88) | 0.016* | 0.054 | 0.485 | |
| SHH | Superficial | 61.85 (48.03, 70.43) | 70.08 (58.81, 79.88) | 83.10 (62.31, 89.33) | 0.209 | <0.01** | 0.015* |
| Middle | 58.80 (37.49, 77.35) | 85.24 (76.23, 89.50) | 89.56 (74.67, 94.12) | <0.01** | <0.01** | 0.217 | |
| Deep | 54.46 (30.97, 73.48) | 29.02 (16.07, 39.74) | 21.29 (11.15, 48.71) | <0.01** | <0.01** | 0.378 | |
| IHH | Superficial | 19.35 (7.18, 68.67) | 3.91 (1.57, 16.18) | 4.81 (1.49, 8.44) | <0.01** | <0.01** | 0.462 |
| Middle | 65.17 (18.08, 80.82) | 20.67 (8.73, 49.38) | 25.13 (9.69, 37.38) | <0.01** | <0.01** | 0.740 | |
| Deep | 6.34 (3.98, 24.63) | 2.95 (0.00, 6.88) | 2.53 (1.88, 5.41) | <0.01** | <0.01** | 0.739 | |
The results are presented as P50 (P25, P75). BMP2 = bone morphogenetic protein 2; BMP4 = bone morphogenetic protein 4; SHH = Sonic hedgehog; IHH = Indian hedgehog; OA = osteoarthritis; KBD = Kashin-Beck disease.
- indicates that multiple comparisons are not required when the rank-sum test of multiple independent samples is not statistically significant.
P < 0.05; ** P < 0.01.
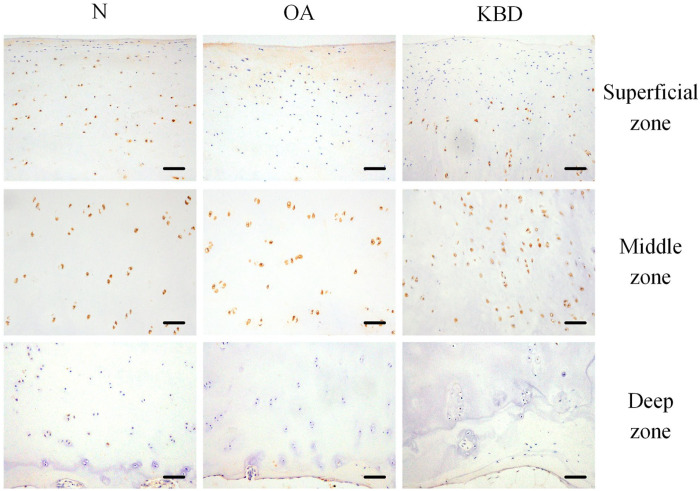
For BMP4, the strongest staining was observed in the middle zone of all 3 groups. However, the staining was much weaker in the superficial zone of the OA group compared with those of the N and KBD groups. The positive staining rate of BMP4 in the superficial zone of the KBD group was significantly higher than that in the OA group, but lower than that in the N group. In addition, the positive staining rate of BMP4 in the superficial zone was significantly lower in the OA group than that in the N group. In the deep zone, a significantly lower rate of BMP4-positive staining was observed in the OA group compared to the N group ( Fig. 2 ; Table 2 ).
Figure 2.
Representative IHC staining of BMP4 in superficial, middle, and deep zones of articular cartilage in normal adults and OA and KBD patients. Scale bar, 20 μm. IHC = immunohistochemistry; BMP4 = bone morphogenetic protein 4; OA = osteoarthritis; KBD = Kashin-Beck disease.
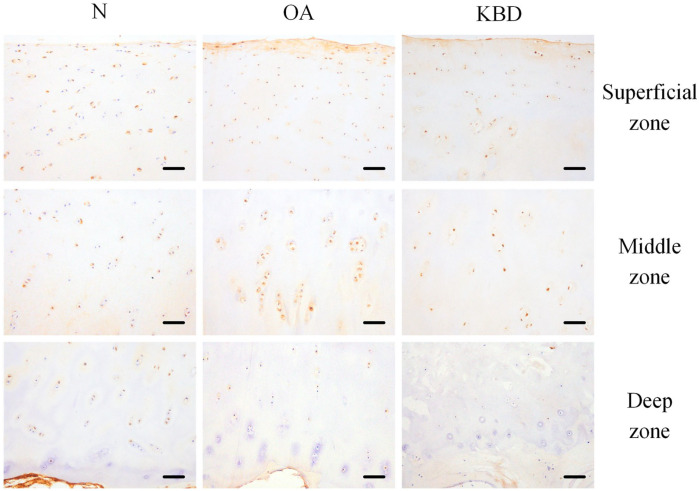
A relatively similar SHH staining was observed in the superficial and middle zones of the cartilage in all 3 groups. The positive staining rate of SHH in the superficial zone of the OA group was significantly lower than that in the KBD group, but higher than that in the N group. The positive staining rate of SHH in the middle zone of the N group was significantly lower than those in the OA and KBD groups. Slightly stronger SHH staining could be seen in the deep zone of the N group when compared with the OA and KBD groups ( Fig. 3 ; Table 2 ).
Figure 3.
Representative IHC staining of SHH in superficial, middle, and deep zones of articular cartilage in normal adults and OA and KBD patients. Scale bar, 20 μm. IHC = immunohistochemistry; SHH = Sonic hedgehog; OA = osteoarthritis; KBD = Kashin-Beck disease.
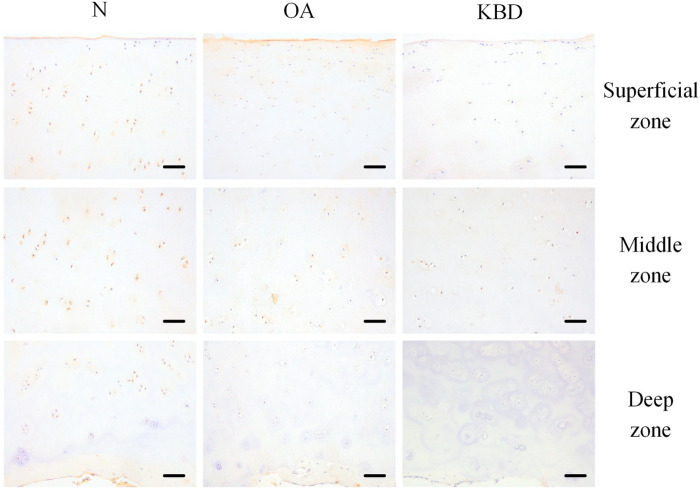
The strongest IHH staining was observed in the middle zones of all 3 groups, but the weakest IHH staining was visible in the deep zones of all 3 groups. The positive staining rates of IHH in all 3 zones were significantly lower in the OA and KBD groups than that in the N group ( Fig. 4 ; Table 2 ).
Figure 4.
Representative IHC staining of IHH in superficial, middle, and deep zones of articular cartilage in normal adults and OA and KBD patients. Scale bar, 20 μm. IHC = immunohistochemistry; IHH = Indian hedgehog; OA = osteoarthritis; KBD = Kashin-Beck disease.
Discussion
Currently, the clinical treatment of OA and KBD patients is limited to analgesics, anti-inflammatory treatment, and other similar methods that are used to improve joint motor function. However, joint replacement surgery is still inevitable because the root of the problem degeneration of the patient’s articular cartilage remains to be solved. Activation of the HH pathway is closely related to the progression of osteoarthropathy.24,25 This study compared the expression levels of the HH pathway proteins BMP2, BMP4, IHH, and SHH in knee articular cartilage tissues from adult OA, KBD patients, and normal people. We hope that it can fill in some of the research gaps in this field, further clarifying the pathogenesis of KBD and OA.
The HH pathway plays key regulatory roles in chondrogenic differentiation. These 4 proteins of the HH pathway were selected for detection in this study because they interact with each other. Transfection of the IHH gene into bone marrow–derived stromal cells (BMSCs) has been found to promote chondrogenic differentiation and inhibit cartilage aging and osteogenesis. SHH can elevate the expression of cartilage markers Sox9 and collagen II, 26 and establish an NKX3.2/SOX9 autoregulatory loop maintained by BMP signaling to induce somatic chondrogenesis.27,28 The differentiation from BMSCs to chondrocytes was promoted via activation of the SHH-related pathway. 29 In addition, SHH is also necessary for BMP2 activation in vertebrate limbs.30,31 Meanwhile, BMP signaling is necessary for the growth and differentiation of cartilage.32,33 Moreover, BMP signal transduction modulates IHH expression levels and works in parallel with IHH to regulate chondrocyte proliferation and hypertrophic differentiation. 21 In our results, expression of BMP2, BMP4, SHH, and IHH in the deep zone was lower in the OA and KBD groups than that in the N group. Expression of BMP4 and IHH was significantly lower in the OA and KBD groups than that in the N group in the middle zone. The expression of BMP2 in the deep zone and BMP4 in superficial and deep zones was also lower in the OA and KBD groups than that in the N group. The deeper the KBD cartilage tissue zones, the more serious and typical the pathological changes. 34 All the above indicate that normal body physiology process was adversely affected when OA and KBD occur. The expression of BMP 2/4 was hindered. Furthermore, as we have already mentioned before, BMP signal transduction modulates IHH and SHH levels, and this may be why their levels were similarly significantly lower in the KBD and OA groups than that in the N group.
Besides, an interesting observation in the results also caught our attention. SHH staining appeared to be changed in an opposite direction as IHH on the whole. In the surface and middle zones, the positive staining rate of SHH in the OA and KBD groups was higher than that in the N group, while for IHH, the positive staining rates in OA and KBD groups were lower than that in the N group. We suspect that there is possibly a compensatory effect of SHH and IHH. In cartilage and joint cavity effusion from OA patients, IHH expression was significantly enhanced. 17 The enhanced levels of IHH were positively correlated with the expression of genes MMP-13 and COL10 related to chondrocyte hypertrophy, which makes the cartilage calcify and gradually lose normal function. 17 Previous studies on SHH have mainly focused on its role in embryonic development, 31 with few studies on its mechanism in cartilage damage of OA and KBD. However, both SHH and IHH belong to the vertebrate HH family, and a large part of sequence identity between SHH and IHH was observed at the gene and protein level. 35 This might make SHH and IHH have similar functions. In this study, IHH showed the opposite results from previous studies. Meanwhile, the changes of SHH and IHH on the whole were opposite, to a certain extent. This phenomenon may be caused by the reciprocal compensatory mechanism between the expressions of SHH and IHH after cartilage injury, which may be further confirmed in future studies.
There are some limitations of this study that need to be acknowledged. First, as the existing cases of KBD are rare and funding for it from various institutions is very low, the sample size is difficult to enlarge in this study, and the experimental results may be biased. Therefore, it is necessary to carry out experiments with a larger sample size to verify the accuracy of these experimental conclusions. Second, IHC staining consists of several steps, and many factors may have influenced the IHC process, resulting in image noise and some slight tissue overlapping, and ultimately likely affecting the results. Maybe more repetition of the experiment can reduce the error of the results. In conclusion, this study found abnormal expression of the HH pathway proteins BMP2, BMP4, SHH, and IHH in OA and KBD cartilage. And there is possibly a compensatory effect of SHH and IHH. These findings can provide a new theoretical and experimental basis for future study in the pathogenesis of OA and KBD; it can also provide targeted therapeutic solutions for the prevention and treatment of diseases.
Footnotes
Author Contributions: All authors participated in drafting the article and critically modifying the important content of knowledge, with all authors endorsing the final version. Deng, Xiao, and Chilufya had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study conception and design: Han. Acquisition of data: Deng, Liu, Lv, Lei, Wang, Zhang. Analysis and interpretation of data: Deng, Chilufya, Guo, Qiao, Xiao, Zhao. Drafting the article or revising it critically for important intellectual content: Deng, Xiao, Chilufya, Qu, Han. Final approval of the version of the article to be published: Deng, Xiao, Chilufya, Qiao, Lv, Guo, Lei, Liu, Zhao, Zhang, Wang, Han, Qu.
Acknowledgments and Funding: We thank Zengtie Zhang for his support and cooperation in the staining of the cartilage slices. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (81872567). The study sponsors were not involved in the study design, data collection, analysis or interpretation, or in the writing of the manuscript, neither did they affect the decision to submit the manuscript for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Human Ethics Committee of the Xi’an Jiaotong University.
ORCID iDs: Mumba Mulutula Chilufya  https://orcid.org/0000-0001-7812-022X
https://orcid.org/0000-0001-7812-022X
Lichun Qiao  https://orcid.org/0000-0002-6739-0846
https://orcid.org/0000-0002-6739-0846
References
- 1. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1): 33-42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 2. Cao J, Li S, Shi Z, Yue Y, Sun J, Chen J, et al. Articular cartilage metabolism in patients with Kashin-Beck Disease: an endemic osteoarthropathy in China. Osteoarthritis Cartilage. 2008;16(6): 680-8. doi: 10.1016/j.joca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3. Guo X, Ma WJ, Zhang F, Ren FL, Qu CJ, Lammi MJ. Recent advances in the research of an endemic osteochondropathy in China: Kashin-Beck disease. Osteoarthritis Cartilage. 2014;22(11): 1774-83. doi: 10.1016/j.joca.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 4. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60(2): 243-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Prevalence and burden of osteoarthritis: results from a population survey in Norway. J Rheumatol. 2008;35(4): 677-84. [PubMed] [Google Scholar]
- 7. Fang H, Guo X, Farooq U, Xia C, Dong R. Development and validation of a quality of life instrument for Kashin-Beck disease: an endemic osteoarthritis in China. Osteoarthritis Cartilage. 2012;20(7): 630-7. [DOI] [PubMed] [Google Scholar]
- 8. Farooq U, Guo X, Chuang LH, Fang H, Zhuang GH, Xia CT. Measuring health-related quality of life in Kashin–Beck disease using EQ-5D. Qual Life Res. 2011;20(3): 425-9. [DOI] [PubMed] [Google Scholar]
- 9. Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24(1): 1-12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10. Yao JQ, Seedhom BB. Mechanical conditioning of articular cartilage to prevalent stresses. Br J Rheumatol. 1993;32(11): 956-65. doi: 10.1093/rheumatology/32.11.956. [DOI] [PubMed] [Google Scholar]
- 11. Lapadula G, Iannone F. Metabolic activity of chondrocytes in human osteoarthritis as a result of cell-extracellular matrix interactions. Semin Arthritis Rheum. 2005;34(6 Suppl 2): 9-12. doi: 10.1016/j.semarthrit.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12. Atwood SX, Chang AL, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199(2): 193-7. doi: 10.1083/jcb.201207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buckland J. Osteoarthritis: blocking hedgehog signaling might have therapeutic potential in OA. Nat Rev Rheumatol. 2010;6(2): 61. doi: 10.1038/nrrheum.2009.270. [DOI] [PubMed] [Google Scholar]
- 14. Rockel JS, Yu C, Whetstone H, Craft AM, Reilly K, Ma H, et al. Hedgehog inhibits β-catenin activity in synovial joint development and osteoarthritis. J Clin Invest. 2016;126(5):1649-63. doi: 10.1172/JCI80205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128(24): 5099-108. [DOI] [PubMed] [Google Scholar]
- 16. Wu Q, Zhang Y, Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276(38): 35290-6. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 17. Wei F, Zhou J, Wei X, Zhang J, Fleming BC, Terek R, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthr Cartil. 2012;20(7):755-63. doi: 10.1016/j.joca.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murtaugh LC, Zeng L, Chyung JH, Lassar AB. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev Cell. 2001;1(3): 411-22. doi: 10.1016/s1534-5807(01)00039-9. [DOI] [PubMed] [Google Scholar]
- 19. Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1-48. doi: 10.1016/s0070-2153(06)76001-x. [DOI] [PubMed] [Google Scholar]
- 20. Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172(1): 126-38. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 21. Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, et al. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128(22):4523-34. [DOI] [PubMed] [Google Scholar]
- 22. Xiong G. Diagnostic, clinical and radiological characteristics of Kashin-Beck disease in Shaanxi Province, PR China. Int Orthop. 2001;25(3): 147-50. doi: 10.1007/s002640100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ilana A. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Aust J Physiotherapy. 2009;55(3): 213. dio: 10.1016/S0004-9514(09)70088-1. [DOI] [PubMed] [Google Scholar]
- 24. Li R, Cai L, Hu CM, Wu TN, Li J. Expression of hedgehog signal pathway in articular cartilage is associated with the severity of cartilage damage in rats with adjuvant-induced arthritis. J Inflamm. 2015;12:24. doi: 10.1186/s12950-015-0072-5/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15(12):1421-5. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 26. Warzecha J, Göttig S, Brüning C, Lindhorst E, Arabmothlagh M, Kurth A. Sonic hedgehog protein promotes proliferation and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. J Orthop Sci. 2006;11(5): 491-6. doi: 10.1007/s00776-006-1058-1. [DOI] [PubMed] [Google Scholar]
- 27. Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16(15): 1990-2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawato Y, Hirao M, Ebina K, Shi K, Hashimoto J, Honjo Y, et al. Nkx3.2 promotes primary chondrogenic differentiation by upregulating Col2a1 transcription. PLoS ONE. 2012;7(4):e34703. doi: 10.1371/journal.pone.0034703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Wei G, Wang X, Liu DH, Deng RD, Li H, et al. Targeting of the Sonic Hedgehog pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol Pharm Bull. 2012;35(8):1328-35. doi: 10.1248/bpb.b12-00265. [DOI] [PubMed] [Google Scholar]
- 30. Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C, et al. A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signalling and Bmp signalling. Development. 2000;127(7):1337-48. [DOI] [PubMed] [Google Scholar]
- 31. Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12(10):1248-56. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 32. Pathi S, Rutenberg JB, Johnson RL, Vortkamp A. Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev Biol. 1999;209(2): 239-53. doi: 10.1006/dbio.1998.9181. [DOI] [PubMed] [Google Scholar]
- 33. Liu PC, Liu K, Liu JF, Xia K, Chen LY, Wu X. Transfection of the IHH gene into rabbit BMSCs in a simulated microgravity environment promotes chondrogenic differentiation and inhibits cartilage aging. Oncotarget. 2016;7(39): 62873-85. doi: 10.18632/oncotarget.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, He Y, Zhang D, Zhang M, Wang M, Zhang Y, et al. Death of chondrocytes in Kashin-Beck disease: apoptosis, necrosis or necroptosis? Int J Exp Pathol. 2018;99(6):312-22. doi: 10.1111/iep.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereira J, Johnson WE, O’Brien SJ, Jarvis ED, Zhang G, Gilbert MT, et al. Evolutionary genomics and adaptive evolution of the Hedgehog gene family (Shh, Ihh and Dhh) in vertebrates. PLoS ONE. 2014;9(12):e74132. doi: 10.1371/journal.pone.0074132. [DOI] [PMC free article] [PubMed] [Google Scholar]