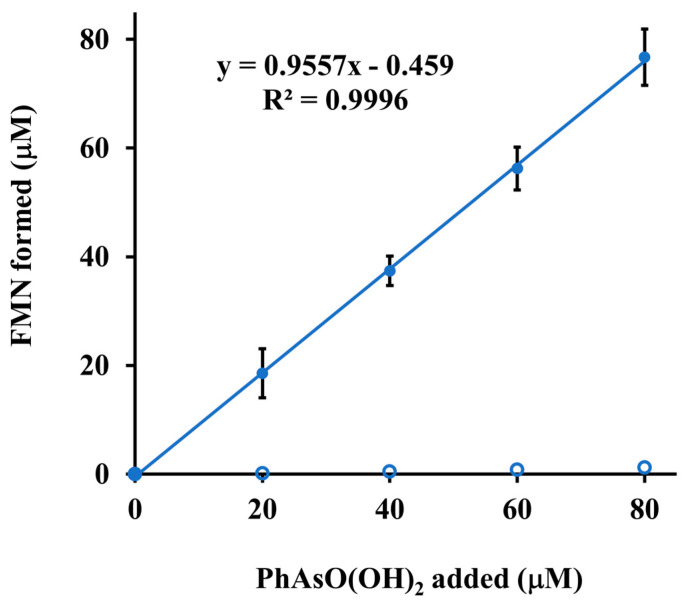
Figure 3.
ArsH-catalyzed oxidation of reduced FMN by phenylarsonic acid. A sealed 1 cm spectrophotometric cuvette contained 1 mL of argon-purged 0.1 M sodium phosphate buffer, pH 7.0, with 100 μM FMN. 205 nM ArsH was either present (solid circles) or absent (open circles). After reduction of FMN with dithionite, measurement was performed by adding successive 20 μM portions of phenylarsonic acid. The resulting absorbance at 450 nm was read after attaining a constant level (within about 5 min). All A450 readings were corrected by subtracting the initial absorbance and converted to concentrations using an absorbance coefficient of 12,200 M−1 cm−1. The value of the slope of the straight line fitted to the data points indicates that the stoichiometric ratio of FMN produced to phenylarsonic acid added is 1:1. Each data point is mean of triplicate determinations ± SD.