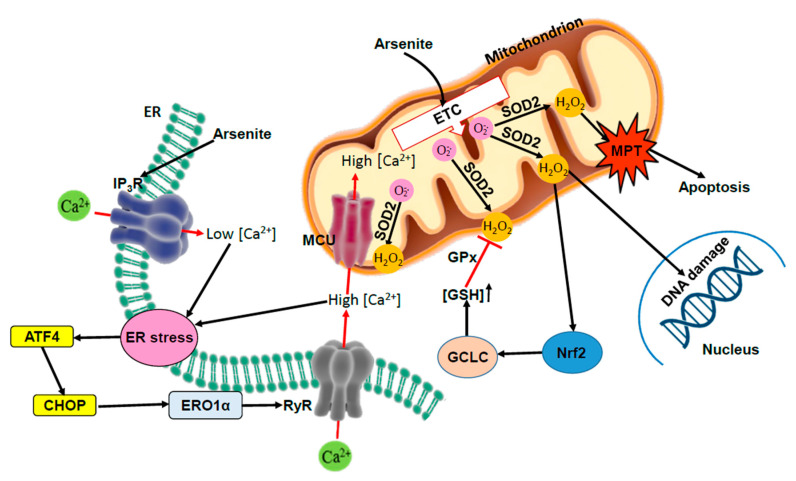
Figure 1.
Crosstalk between arsenite-induced mitochondrial ROS, ER stress, and Nrf2. Arsenite promotes mitoO2•− formation via a mechanism requiring interactions with the mitochondrial respiratory chain and an accumulation of Ca2+ in these organelles. The metalloid initially stimulates Ca2+ release from the IP3R, which, although not directly taken up by the mitochondria, nevertheless contributes to this event via RyR stimulation. Indeed, due to the close apposition with the mitochondria, only the fraction of Ca2+ the RyR can be taken up by the mitochondria. Cells uniquely expressing the IP3R, in which these channels are in close contact with the mitochondria, failed to generate mitoO2•− in response to arsenite. RyR activation was regulated by ERO1α and the resulting mitochondrial accumulation of Ca2+ was critical for the formation of mitoO2•. In this perspective, while the ER stress response appears upstream to mitoO2•− formation, it is nevertheless reasonable to predict that persistent mitoO2•−-derived H2O2 promotes mitochondrial dysfunction and toxicity. The early ER stress response was also critically connected through mitoO2• formation with the triggering of the Nrf2 cytoprotective signaling, which indeed significantly mitigated and delayed the onset of MPT-dependent apoptosis.