Abstract
Objectives:
We aim to compare transcaval and transaxillary artery access for TAVR at experienced medical centers in contemporary practice.
Background:
There are no systematic comparisons of transcaval and transaxillary TAVR access routes.
Methods:
Eight experienced centers contributed local data collected for the TVT Registry between 2017 and 2020. Outcomes after transcaval and axillary/subclavian (transaxillary) access were adjusted for baseline imbalances using doubly robust (inverse-propensity-weighting plus regression) estimation and compared.
Results:
Transcaval access was used in 238, transaxillary in 106, and for comparison transfemoral in 7132. Risk profiles were higher among patients selected for nonfemoral access, but similar among patients requiring transcaval and transaxillary access.
Stroke or TIA were five-fold less common after transcaval than transaxillary [2.5% vs 13.2%, OR 0.20 (0.06–0.72), p=0.014], compared with transfemoral (1.7%). Major and life-threatening bleeding (VARC-3 ≥ Type-2) were comparable [10.0% vs 13.2%, OR 0.66 (0.26–1.66), p=0.38], compared with transfemoral (3.5%), as was blood transfusion [19.3% vs 21.7%, OR 1.07 (0.49–2.33), p=0.87], compared with transfemoral (7.1%). Vascular complications, ICU and hospital length of stay, and survival were similar between transcaval and transaxillary. More patients were discharged directly home and without stroke or TIA after transcaval than transaxillary (87.8% vs 62.3%, OR 5.19 (2.45–11.0), p<0.001) compared with transfemoral (90.3%).
Conclusions:
Patients undergoing transcaval TAVR had lower rates of stroke and similar bleeding compared with transaxillary access in a contemporary experience from 8 US centers. Both approaches had more complications than transfemoral access. Transcaval TAVR access may offer an attractive option.
Keywords: Transcaval access, Transaxillary access, Subclavian access, Nonfemoral access Alternative access, Percutaneous access
CONDENSED ABSTRACT
We aggregated local data collected for TVT registry between 2017–2020 at eight sites experienced with transcaval and transaxillary access for TAVR. Stroke/TIA were less common after transcaval than transaxillary [2.5% vs 13.2%, OR 0.20 (0.06–0.72), p=0.014], compared with transfemoral (1.7%). Major and life-threatening bleeding (VARC-3≥Type-2) were comparable [10.0% vs 13.2%, OR 0.66 (0.26–1.66), p=0.38], as was blood transfusion [19.3% vs 21.7%, OR 1.07 (0.49–2.33), p=0.87]. Discharge directly home without stroke was more common after transcaval than transaxillary. Other outcomes were similar. Transcaval access confers a lower risk of stroke/TIA, comparable bleeding, and more frequent discharge directly home than transaxillary access.
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) requires large-bore access to the aorta. Nonfemoral access is required when the iliofemoral arteries are too small or too diseased to allow standard percutaneous transfemoral artery access. Nonfemoral access confers a higher risk of procedural complications and mortality (1–5).
The most common nonfemoral access route is percutaneous or surgical transaxillary or transsubclavian (6–12). Transcaval access is a counterintuitive approach to enter the abdominal aorta percutaneously from the adjoining inferior vena cava. After TAVR, the transcaval access port is closed with a nitinol occluder device. Transcaval access relies on interstitial hydrostatic pressure exceeding venous pressure. As a result, blood exiting the aorta tends to return from the retroperitoneal space back into the venous system rather than accumulating as hemorrhage. The technique of transcaval access is reviewed elsewhere (13–15).
The earliest reports of transcaval access mostly included patients with high or prohibitive risk and few access options, were performed in the era of first and second generation transcatheter heart valves (2013–2016) requiring larger introducer sheaths, and were associated with high bleeding rates (16,17) but no late complications(15). With operator and site experience, vascular and bleeding complication rates appear to have declined for most TAVR access routes (18–22).
There are no systematic comparisons of transcaval and transaxillary access routes, nor contemporary assessments of outcomes after transcaval access. We hypothesize that transcaval access would have similar bleeding outcomes compared with transaxillary access by experienced operators, and that by comparison, patients eligible for standard transfemoral access have fewer comorbidities and complications. Based on reports of increased stroke risk of transaxillary compared with transfemoral access (8), we also hypothesized that transcaval access may confer a lower risk of stroke by avoiding instrumentation of the head and neck arteries.
METHODS
Participating sites
Eight sites contributed data from the time period between January 2017 and December 2020. Sites were selected because of experience (TAVR volume (>100 cases per year); service as TAVR proctors; and experience with both transaxillary and transcaval access); as well as prior participation in NHLBI sponsored structural heart IDE trials (n=6), or having expressed interest (n=2). All sites participated without compensation. Outcomes data had already been collected prospectively on all consecutive patients undergoing TAVR, as a condition of CMS reimbursement via the Society of Thoracic Surgeons and American College of Cardiology (ACC) Transcatheter Valve Therapy (STS/ACC TVT) registry [NCT01737528](23,24). Individual site data submitted to this registry for all patients undergoing TAVR at these centers (data collection form version 2.1) were aggregated for central analysis. The ACC National Cardiovascular Data Registry and STS/ACC TVT registry neither endorsed nor participated in the study.
TAVR access routes were selected by the local institutional heart teams based on clinical findings and operator discretion only. Procedures were planned and performed according to local institutional standards. Both subclavian and axillary access routes are combined as “transaxillary” for the purpose of this analysis.
This study includes 36 patients undergoing transcaval TAVR from one site that previously reported 22 transcaval TAVR separately(25).
Ethics
The Emory University Institutional Review Board approved this retrospective study. Participating sites also obtained local ethics board waiver of consent to transmit coded clinical data to Emory University, which served as the data coordinating center.
Data classifications
The following calculations applied to baseline characteristics: Transfusion-corrected hemoglobin drop is calculated as (pre-procedure hemoglobin g/dL) – (post-procedure hemoglobin g/dL) – (units of transfused blood), which assumes that each unit of transfused blood increments hemoglobin by 1g/dL. For example, a patient with admission and discharge hemoglobin values of 12g/dL and 10g/dL would have a hemoglobin drop of 2g/dL irrespective of a 3unit blood transfusion but would have a transfusion-corrected hemoglobin drop of 5g/dL. CHA2DS2-VASc score was calculated with the following deviations: only heart failure in past 2 weeks rather than ever; without regard to prior non-cerebral thromboembolism; and without regard to aortic plaque.
The primary outcomes of interest are bleeding complications and stroke. Outcomes are defined by Valve Academic Research Consortium (VARC-3) definitions (26), with minor deviations. One exception is that life-threatening bleeding (VARC-3 Type 3) was calculated without regard to sustained hypotension, part of the definition that was not captured by TVT registry data collection form v2.1. Another exception is that Acute Kidney Injury (AKI) stage was calculated based on index hospital admission rather than 7 days. Another exception is that TVT registry does not capture modified Rankin Score used to classify stroke as “disabling” or “non-disabling,” although it does capture other indices of disability reported here.
We created a post-hoc patient-oriented composite outcome consisting of survival to discharge, discharge directly to home instead of another institution, and without stroke/TIA.
Statistical Analysis
A propensity score for each patient was estimated using generalized boosted modeling (GBM) (27). Pre-procedure characteristics constituted the explanatory variables in the GBM, and the response was the probability of assignment to transaxillary as opposed to transcaval. In this study, GBMs relied on a composition of tree-based regression models that were built in an iterative fashion; in each iteration, the number of regression trees added to the model increased. The iterative process was terminated when more complex models resulted in greater preoperative imbalance. GBM is a data-adaptive, nonparametric model whose primary advantage over logistic regression is its ability to select preoperative variables and determine whether higher-order terms or interactions are needed. GBM combines many piecewise-constant functions of the covariates to estimate the propensity scores.
Inverse probability of treatment weighting using the propensity score was then used to create a synthetic sample in which the distribution of measured preoperative characteristics was independent of treatment assignment. After fitting the GBM which generated the propensity score weights, the next step was to assess how well the propensity score weights corrected the preoperative between-group imbalances. The assessment was based on the absolute standardized mean difference (ASMD), allows group means and proportions to be compared without being influenced by sample size. ASMD larger than 0.20 indicates that the groups being compared are too different from each other for reliable outcome comparison.
Finally, propensity score analysis was performed by assessing the difference in the weighted outcomes between groups. For dichotomous outcomes, logistic regression models were built, and odds ratios (OR) were estimated. For continuous outcomes, linear regression models were built after the outcome was log transformed. Exponentiation of the regression parameter provided the geometric mean ratio (GMR). Finally, because some of the ASMD were larger than 0.1 (but less than 0.2), we next performed doubly robust estimation, which requires constructing the propensity score weighted regression model with the additional baseline variables, to obtain consistent treatment effects.
Propensity scores were generated by a GBM using the Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG) package and %PS macro (RAND corporation, Santa Monica, CA). The quality of the propensity scores was assessed using accompanying macros provided by RAND. For the outcomes analyses, survey procedures in SAS, such as SURVEYREG SURVEYLOGISTIC, were used to incorporate the propensity score weights.
Subjects were excluded if they underwent multiple separate inpatient TAVR procedure admissions which confound endpoint assessments (n=14, all having undergone transfemoral TAVR). There was no adjustment for multiple comparisons. All tests of hypotheses were two-sided and conducted at a 0.05 level of significance. Analyses used SAS (SAS Institute, Cary, NC) and R (https://www.R-project.org/). Data are expressed as mean (standard deviation); median (1st quartile, 3rd quartile); n (%); or Odds Ratio [95% Confidence Interval], as appropriate.
RESULTS
Sites and patients
Eight sites contributed data on 7539 patients undergoing TAVR during the four-year study period (TABLE 1). 407 underwent nonfemoral TAVR, constituting 5.4% of TAVR procedures. Only 63 of these were transcarotid (n=29) or transthoracic (transapical or transaortic, n=34) and were not evaluated further.
TABLE 1:
Enrollment sites and TAVR access site counts
| Site | All | Transfemoral | Transcaval | Transaxillary | Carotid | Thoracic | Nonfemoral |
|---|---|---|---|---|---|---|---|
| Site 1 | 1439 | 1405 | 34 | 0 | 0 | 0 | 2.4% |
| Site 2 | 1250 | 1193 | 5 | 48 | 1 | 3 | 4.6% |
| Site 3 | 1242 | 1133 | 80 | 7 | 20 | 2 | 8.8% |
| Site 4 | 952 | 889 | 63 | 0 | 0 | 0 | 6.6% |
| Site 5 | 866 | 828 | 15 | 0 | 0 | 23 | 4.4% |
| Site 6 | 728 | 682 | 28 | 14 | 4 | 0 | 6.3% |
| Site 7 | 568 | 536 | 4 | 18 | 4 | 6 | 5.6% |
| Site 8 | 494 | 466 | 9 | 19 | 0 | 0 | 5.7% |
| All | 7539 | 7132 | 238 | 106 | 29 | 34 | 5.4% |
Of the remaining nonfemoral procedures, 238 were transcaval and 106 were transaxillary, constituting 4.5% of the patients undergoing TAVR. Three of 8 sites preferentially performed transaxillary TAVR over transcaval; three sites that preferentially performed transcaval TAVR reported no transaxillary TAVR during the study period. Most (84%) of the transaxillary procedures were percutaneous, and the remainder underwent surgical cut-down. Only 197 (2.7%) of all procedures were coded as employing cerebral embolic protection devices, including 4 transcaval and 2 transaxillary. Including 3.2% withdrawal and loss to follow-up, 91% of patients were available for 30-day follow-up and stroke assessment, evenly distributed among access routes.
TABLE 2 shows unadjusted baseline and procedure characteristics of patients, stratified by access site. Patients undergoing transcaval and transaxillary access tended to have a higher risk profile compared with transfemoral, and similar risk profile compared with each other. Patients undergoing transcaval TAVR more often were women, had moderate sedation, and had longer procedures requiring more contrast and more fluoroscopy, compared with transaxillary. TAVR success was similar across all access approaches. Missing datapoints were infrequent.
TABLE 2.
Baseline and Procedure Characteristics
| All (n=7476) | Missing% | Transfemoral (n=7132) | Transcaval (n=238) | Transaxillary (n=106) | |
|---|---|---|---|---|---|
| BASELINE CHARACTERISTICS | |||||
| Age | 77.6 (9.9) | 0.0% | 77.7 (10.0) | 76.4 (9.1) | 77.2 (8.8) |
| Female Sex | 3330 (44.5%) | 0.0% | 3150 (44.2%) | 134 (56.3%) | 46 (43.4%) * |
| Non-White | 997 (14.4%) | 7.5% | 940 (14.3%) | 49 (20.9%) | 8 (9.1%) * |
| Private Insurance | 3757 (59.0%) | 14.8% | 3603 (59.3%) | 125 (56.3%) | 29 (42.0%) * |
| STS-PROM score | 3.9 (2.4,6.5) | 8.5% | 3.9 (2.4,6.4) | 5.0 (3.2,8.4) | 5.6 (4.0,8.3) |
| Tobacco Use | 509 (6.8%) | 0.1% | 439 (6.2%) | 45 (18.9%) | 25 (23.6%) |
| Body mass index (kg/m2) | 29.2 (6.7) | 1.6% | 29.3 (6.7) | 26.4 (6.1) | 28.0 (8.8) |
| Diabetes | 2946 (39.4%) | 0.1% | 2810 (39.4%) | 90 (37.8%) | 46 (43.4%) |
| Hypertension | 6758 (90.4%) | 0.0% | 6437 (90.3%) | 223 (94.1%) | 98 (92.5%) |
| ESRD on Dialysis | 382 (5.1%) | 0.0% | 355 (5.0%) | 21 (8.8%) | 6 (5.7%) |
| Heart Failure in preceding 2 weeks | 5942 (79.6%) | 0.1% | 5663 (79.5%) | 192 (80.7%) | 87 (82.1%) † |
| NYHA Class III or IV | 4022 (59.2%) | 9.2% | 3811 (58.9%) | 140 (60.1%) | 71 (84.5%) † |
| Atrial Fibrillation | 2685 (36.0%) | 0.2% | 2567 (36.0%) | 82 (34.6%) | 36 (34.0%) |
| CHA2DS2-Vasc Score | 4.0 (1.2) | 0.6% | 3.9 (1.2) | 4.6 (1.1) | 4.6 (1.0) |
| Prior Stroke | 803 (10.7%) | 0.1% | 745 (10.5%) | 41 (17.2%) | 17 (16.0%) |
| Prior TIA | 528 (7.1%) | 0.2% | 497 (7.0%) | 18 (7.6%) | 13 (12.3%) |
| Prior Stroke or TIA | 1200 (16.1%) | 0.2% | 1122 (15.8%) | 52 (21.8%) | 26 (24.5%) |
| Carotid Artery Stenosis | 945 (20.7%) | 38.9% | 848 (19.7%) | 74 (37.9%) | 23 (35.4%) |
| Prior myocardial infarction | 1294 (17.3%) | 0.2% | 1209 (17.0%) | 58 (24.4%) | 27 (25.7%) |
| Prior PCI | 1907 (25.5%) | 0.1% | 1790 (25.1%) | 76 (31.9%) | 41 (38.7%) |
| Prior CABG | 1200 (16.1%) | 0.1% | 1115 (15.7%) | 64 (26.9%) | 21 (19.8%) |
| Chronic lung disease, moderate-severe | 1339 (18.1%) | 1.0% | 1208 (17.1%) | 96 (40.3%) | 35 (34.0%) |
| Porcelain aorta | 221 (3.0%) | 0.2% | 198 (2.8%) | 11 (4.6%) | 12 (11.3%) * |
| Oxygen used at home | 511 (6.8%) | 0.0% | 461 (6.5%) | 35 (14.7%) | 15 (14.2%) |
| KCQ12 Overall Summary Score | 49.9 (24.9) | 12.6% | 50.0 (24.9) | 47.3 (25.2) | 44.7 (23.6) |
| eGFR (mL/min/1.73m2) | 60.9 (24.1) | 0.0% | 61.0 (24.0) | 60.1 (25.6) | 55.2 (25.8) |
| Hemoglobin, baseline (g/dL) | 12.1 (2.0) | 0.3% | 12.1 (2.0) | 11.7 (1.9) | 11.5 (2.0) |
| 5m walk test: Not performed / Unable / Performed | 19 / 10 / 71% | 7.4% | 19 / 10 / 71% | 15 / 16 / 69% | 28 / 17 / 55% * |
| 5m walk time (s) | 6.3 (5.0, 8.0) | 7.4% | 6.3 (5.0, 8.0) | 6.7 (5.7, 8.7) | 7.0 (6.0, 8.7) |
| Body mass index < 20 kg/m2 | 338 (4.4%) | 1.6% | 298 (4.1%) | 29 (12%) | 11 (10%) |
| Albumin < 3.3g/dL | 854 (14%) | 17% | 792 (13%) | 46 (23%) | 16 (18%) |
| Left ventricular ejection fraction | 54.5% (50.0,63.0) | 1.1% | 58.0% (50.0,63.0) | 58.0% (43.0,60.0) | 57.0% (43.0,63.0) |
| Aortic valve mean gradient | 41.8 (14.6) | 3.6% | 41.9 (14.6) | 40.1 (13.5) | 41.1 (13.9) |
| TAVR PROCEDURE CHARACTERISTICS | |||||
| Valve-in-Valve TAVR | 602 (8.7%) | 7.6% | 574 (8.7%) | 21 (9.0%) | 7 (8.0%) |
| Moderate sedation | 5922 (79.3%) | 0.2% | 5799 (81.4%) | 108 (45.4%) | 15 (14.2%) † |
| General anesthesia | 1,521 (20%) | 0.2% | 1,301 (18%) | 130 (55%) | 90 (84%) † |
| TAVR Introducer Sheath nominal diameter (Fr) | 14 (14, 16) | 0.0% | 14 (14, 16) | 14 (14, 14) | 14 (14, 16) |
| Fluoroscopy time | 15.3 (11.0,22.0) | 1.5% | 15.0 (10.8,21.0) | 33.6 (26.1,45.4) | 24.9 (16.5,34.9) † |
| Fluoroscopy dose | 901±1347 | 3.0% | 875 (1340) | 1630 (1194) | 996 (1600) † |
| Contrast volume | 89 (61) | 1.6% | 88 (59) | 131 (74) | 101 (95) † |
| Conversion to open heart surgery | 15 (0.2%) | 0.1% | 13 (0.2%) | 2 (0.8%) | 0 (0.0%) |
| VARC-3 TAVR technical success | 7373 (98.8%) | 0.1% | 7033 (98.7%) | 235 (99.2%) | 105 (99.1%) |
p<0.05 comparing transaxillary with transcaval
p<0.01 comparing transaxillary with transcaval.
Continuous outcomes are reported using mean (standard deviation) or median (1st quartile, 3rd quartile). Categorical variables are summarized using count (percentage). STS-PROM = Society of Thoracic Surgery Predicted Risk Of 30-day Mortality after surgical aortic valve replacement; ESRD = End-stage renal disease; NYHA = New York Heart Association heart failure classification; CHA2DS2-Vasc = congestive heart failure, hypertension, age ≥75 (doubled), diabetes mellitus, prior stroke or transient ischemic attack (doubled), vascular disease, age 65–74, female; TIA = Transient ischemic attack; PCI = Percutaneous coronary intervention; CABG = Coronary artery bypass grafting; KCCQ = Kansas City Cardiomyopathy Questionnaire; eGFR = Estimated glomerular filtration rate; VARC-3 = Third valve academic research consortium classification
Complications and outcomes
TABLE 3 shows inpatient or 30-day outcomes before adjustment for baseline imbalances. FIGURE 1 and CENTRAL ILLUSTRATION show outcomes after propensity-score weighted adjustment, using doubly robust estimators, to correct for these imbalances.
TABLE 3:
Unadjusted outcomes
| OUTCOMES | All (n=7476) | Missing% | Transfemoral (n=7132) | Transcaval (n=238) | Transaxillary (n=106) |
|---|---|---|---|---|---|
| BLEEDING-Related | |||||
| Bleeding Any (VARC-3 Types 1–4) | 380 (5.1%) | 0.0% | 330 (4.6%) | 31 (13.0%) | 19 (17.9%) |
| Bleeding Major (VARC-3 Type 2) | 166 (2.2%) | 0.0% | 144 (2.0%) | 14 (5.9%) | 8 (7.5%) |
| Bleeding Life-threatening (VARC-3 Type 3) | 123 (1.6%) | 0.0% | 107 (1.5%) | 10 (4.2%) | 6 (5.7%) |
| Bleeding Major or Life-threatening (VARC-3 Types 2–4) | 289 (3.9%) | 0.0% | 251 (3.5%) | 24 (10.1%) | 14 (13.2%) |
| Blood transfusion | 572 (7.7%) | 0.2% | 503 (7.1%) | 46 (19.3%) | 23 (21.7%) |
| Units of blood if transfused | 2.0 (1.0,3.0) | 0.0% | 2.0 (1.0,3.0) | 2.0 (1.0,4.0) | 2.0 (1.0,3.0) |
| Hemoglobin Drop | 1.5 (1.7) | 0.4% | 1.5 (1.6) | 2.0 (2.0) | 2.5 (1.8) |
| Hemoglobin Drop, Transfusion-corrected | 1.8 (2.3) | 0.5% | 1.7 (2.2) | 2.6 (3.2) | 3.2 (3.2) |
| VASCULAR and NEUROVASCULAR | |||||
| Vascular complications, major | 53 (0.7%) | 0.0% | 46 (0.6%) | 6 (2.5%) | 1 (0.9%) |
| Vascular surgery, unplanned | 199 (2.7%) | 0.0% | 177 (2.5%) | 13 (5.5%) | 9 (8.5%) |
| Stroke or TIA, in-hospital | 141 (1.9%) | 0.0% | 121 (1.7%) | 6 (2.5%) | 14 (13.2%) |
| Stroke or TIA, at 30d | 173 (2.3%) | 9.3% | 152 (2.1%) | 7 (2.9%) | 14 (13.2%) |
| ACUTE KIDNEY INJURY STAGE | |||||
| Stage 0/1/2/3/4 (%) | 93/2.0 /0.6/3.5/0.5 % | 1.8% | 94/1.9/0.6/3.4/0.5/0.5% | 93/2.6/0.4/3.9/0/0% | 84/6.7/0/8.6/1/0.9% |
| Stage ≥ 2 | 337 (4.6%) | 1.8% | 317 (4.5%) | 10 (4.3%) | 10 (9.5%) |
| DISCHARGE | |||||
| ICU hours | 7.9 (0.0,27.3) | 0.3% | 6.4 (0.0,27.0) | 20.7 (0.0,34.0) | 27.1 (19.5,67.0) |
| Length-of-stay (survivors to discharge, d) | 4.2 (9.6) | 0.0% | 4.1 (9.8) | 4.8 (5.6) | 6.5 (6.4) |
| Survived to discharge | 7384 (98.8%) | 0.0% | 7053 (98.9%) | 229 (96.2%) | 102 (96.2%) |
| Discharge directly to home | 6767 (91.6%) | 1.2% | 6484 (91.9%) | 212 (92.6%) | 71 (69.6%) |
| Discharged alive, directly to home, and without stroke or TIA | 6712 (89.8%) | 0.0% | 6437 (90.3%) | 209 (87.8%) | 66 (62.3%) |
| AFTER-DISCHARGE | |||||
| 30day Death | 139 (1.9%) | 0.0% | 118 (1.7%) | 14 (5.9%) | 7 (6.6%) |
| One year Death | 445 (6.0%) | 0.0% | 414 (5.8%) | 21 (8.8%) | 10 (9.4%) |
One-year survival follow-up was 356 (38, 379) days after the index procedure; 670 (9%) of subjects were missing. Continuous outcomes are reported using mean (standard deviation) or median (1st quartile, 3rd quartile). Categorical variables are summarized using count (percentage). ICU = Intensive Care Unit; LOS = Length of Stay; Transfusion-corrected hemoglobin drop is calculated as (baseline – discharge hemoglobin) + (units of PRBC transfused).
Figure 1.
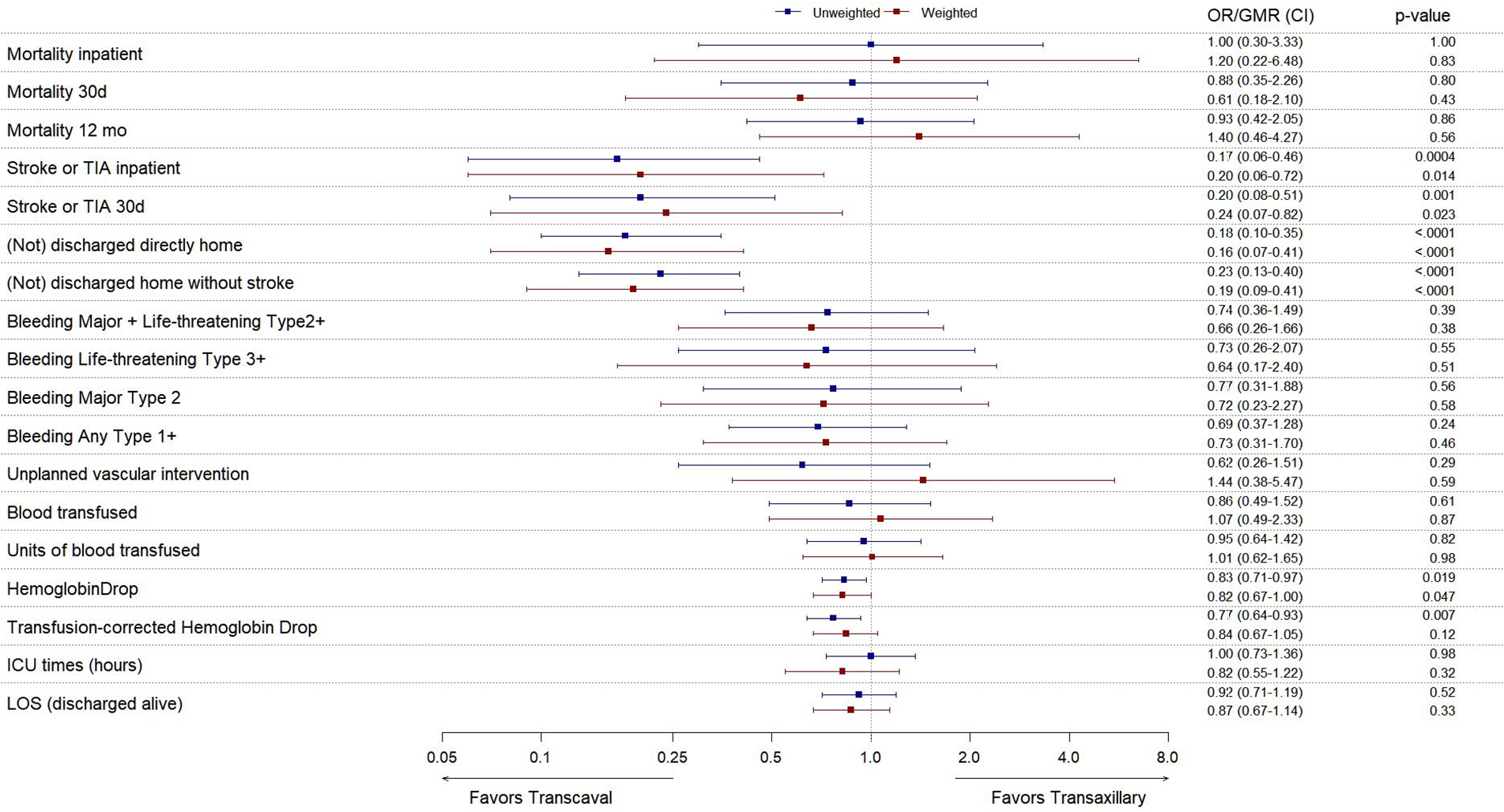
Outcomes before- and after adjustment for baseline imbalances.
Forest plot of relative outcomes of transcaval versus transaxillary access, expressed as OR (Odds Ratio) for parametric data and as GMR (Geometric Mean Ratio) for continuous data, with 95% CI (confidence intervals). Values with confidence intervals that do not cross OR/GMR=1.0 are considered statistically significant. Plots show unweighted (blue) and inverse propensity-score-weighted (red) outcomes. ICU = Intensive Care Unit; LOS = Length of Stay; Corrected hemoglobin drop = (baseline – discharge hemoglobin) + (units of PRBC transfused).
Central Illustration.
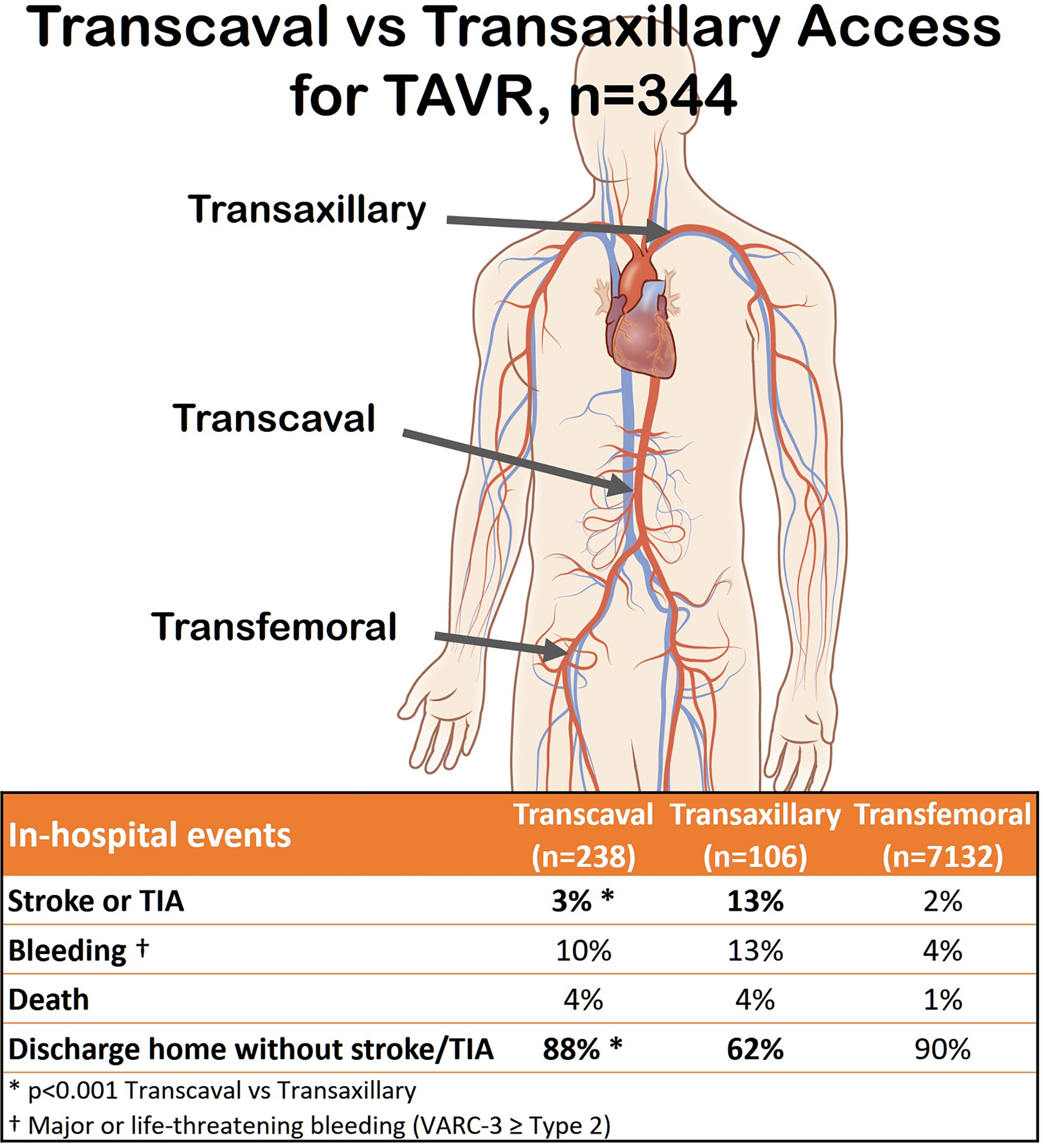
Key outcomes after transcaval versus transaxillary access in contemporary practice.
Nonfemoral access (transcaval and transaxillary) conferred worse outcomes than transfemoral access, including worse bleeding, vascular, complications, stroke or TIA, ICU and hospital length of stay, 30-day mortality, and one year (follow-up 356 (38, 379) days) mortality. Indeed the doubly-robust estimator approach failed to correct for baseline imbalances comparing transfemoral- with nonfemoral access techniques.
Comparing transcaval with transaxillary, bleeding and vascular complications were comparable on all measures except hemoglobin drop, which slightly favored transcaval.
Stroke or TIA were significantly less common after transcaval than transaxillary access, both before and after inverse-propensity-weighting (2.5% vs 13.2%, OR 0.20 (0.06–0.72), p=0.014). Use of cerebral embolic protection devices was sparse, and none were used among patients suffering inpatient stroke or TIA. Six (2.5%) suffered neurovascular events after transcaval and 14 (13.2%) after transaxillary access. All were confirmed by neuroimaging and 94% by a neurology specialist. One was a TIA, 78% had symptoms lasting > 24 hours, 79% had a persistent cognitive, social, or physical disability; one was fatal. These appeared comparably distributed among access routes.
Significantly more patients were discharged directly to home (versus a nursing facility or hospice) after transcaval than after transaxillary access (92.6% vs 69.6%, OR 6.1, CI 2.4–15, p<0.0001). While mortality measures were comparable, the patient-oriented composite of survival to discharge, to home, without stroke or TIA was significantly lower after transaxillary than transcaval access (87.8% vs 62.3%, OR 5.2, CI 2.4–11, p<0.0001), and compared with transfemoral (90.3%). Of the patients not discharged directly home after transaxillary TAVR, 44% went to a nursing home and the rest to an extended care facility.
Outcomes were similar comparing transaxillary cut down versus percutaneous on all measures (data not shown) except duration of ICU care (48 (28, 79) hours versus 25 (3, 66), p=0.013). Similarly, transaxillary outcomes were similar comparing participating sites performing above- and below-median volumes of transaxillary access (data not shown). Moreover, outcomes were similar comparing study periods 2017–2018 versus 2019–2020 (data not shown).
DISCUSSION
This observational study suggests that transcaval access had a lower stroke risk and comparable bleeding and vascular complications compared with transaxillary access, in contemporary practice among experienced operators. In this report transcaval access conferred a transfemoral-like low risk of stroke. The reduced stroke risk persisted after adjustment for differences in baseline characteristics that might have influenced non-random treatment allocation. The proposed mechanism of stroke after transaxillary access is sheath instrumentation of atherosclerotic head and neck vessels. By contrast, transfemoral and transcaval procedures traverse the aorta without instrumenting the head and neck vessels. We infer, provocatively, that transcaval TAVR may prove the percutaneous nonfemoral access of choice; this requires more real-world experience in centers in and outside the USA.
TABLE 4 summarizes our study in the context of other reported studies of transcaval and transaxillary access by time period and risk profile, along with results of pivotal commercial studies in low-risk patients for comparison. Our study shows a comparatively lower risk of bleeding and stroke after transcaval access than earlier reports. Importantly, our observed rate of stroke after transaxillary access is substantially higher than previous reports of stroke after transaxillary and subclavian access.
TABLE 4.
Literature Comparison
| Author | Enrollment Period | Access | Patients | STS 30-day PROM | 30 Day Death | 30 Day Stroke+TIA | LT or Major Bleeding (VARC-3 ≥Type-2) | Major Vascular Complication | Length of Stay (d) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| PIVOTAL LOW-RISK TAVR FOR COMPARISON | |||||||||
|
| |||||||||
| Mack(20) | 2016–2017 | Transfemoral | 496 | 1.9 ± 0.7 | 0.40% | 0.60% | 3.60% | 2.20% | 3 (2,3) |
| Popma(19) | 2016–2018 | Transfemoral & Transaxillary (n=4) | 725 | 1.9 ± 0.7 | 0.50% | 3.40% | 2.40% | 3.80% | — |
| This study | 2017–2020 | Transfemoral | 7132 | 3.9 (2.4, 6.4) | 1.7% | 2.1% | 3.5% | 0.6% | 4.1 ± 9.7 |
|
| |||||||||
| TRANSCAVAL ACCESS Previous Reports | |||||||||
|
| |||||||||
| Greenbaum(16) | 2014–2016 | Transcaval | 100 | 9.6 ± 6.3 | 8% | 5% | 18% | 19% | 4 (IQR 2,6) |
| Costa(17) | 2014–2019 | Transcaval | 50 | 8.6 ± 6.7 | 4% | 2% | 4% + | 14% | 4 (IQR 3,7) |
| Barbash(33) | 2019–2021 | Transcaval | 20 | 4.1 (2.9, 4.9) | 10% | 10% | 10% | 5% * | 6 (IQR 4,7) |
| Weighted mean: | 8.7 | 7.0% | 4.7% | 12.9% | 15.9% | 4.2 | |||
| This study | 2017–2020 | Transcaval | 238 | 5.0 (3.2, 8.4) | 5.9% | 2.9% | 10.1% | 2.5% | 4.8 ± 5.5 |
|
| |||||||||
| TRANSAXILLARY ACCESS Previous Reports | |||||||||
|
| |||||||||
| Schäfer(6) | 2010–2016 | Transaxillary | 100 | 7.2 ± 5.2 | 6.0% | 1.0% | 3.0% | 11% * | 7.9 ± 4.3 |
| Gleason(7) | 2010–2014 | Transaxillary | 202 | 9.7 ± 5.9 | 5.4% | 7.5% | 39% | 12% | 8.3 ± 6.5 |
| Dahle(8) | 2010–2018 | Transaxillary | 1,180 | 7.7 ± 5.8 | 5.4% | 6.3% | N.A. | 2.5% | 3 (IQR 2,5) |
| Debry(9) | 2010–2018 | Transaxillary | 113 | 5.8 (3.9–8.6) | 5.5% | 3.5% | 3.6% | 8.0% | 9 (IQR 6,11) |
| Kirker(10) | 2015–2019 | Transaxillary | 1576 | 7.0 ± 5.39 | 5.2% | 7.4% | N.A. | 2.2% | 3 (IQR 2,5) |
| Amer(11) | 2016–2018 | Transaxillary | 38 | 12.0 ± 9.5 | 2.6% | 7.9% | 2.6% | 0.0% | 5.11 ± 4.77 |
| Ooms(12) | 2018–2020 | Transaxillary | 35 | 4.3 ± 1.8 | 2.9% | 5.7% | 11% | 17% | 5 (IQR 3,8) |
| Weighted mean: | 7.4 | 5.3% | 6.7% | 18.7% | 3.5% | 3.7 | |||
| This study | 2017–2020 | Transaxillary | 106 | 5.7 (4.0, 8.5) | 6.6% | 13.0% | 13.2% | 0.9% | 6.2 ± 6.0 |
Data are expressed as mean ± standard deviation or median (1st, 3rd quartile).
Does not include major (VARC3 type 2) bleeding
Includes bailout stent-graft implantation
The key challenge to the current report is determining whether the comparisons between transcaval and transaxillary access are valid. First, are the patients equally sick and equally predisposed to complications? While the patients were not randomly allocated to access route, we used propensity score weighting to correct for between-group pre-procedure imbalances. All baseline variables had a standardized difference less than 0.2. Variables with a standardized difference larger than 0.1 (and less than 0.2) were included in a regression model fit with the propensity score weights to obtain doubly robust estimators.
Second, does operator or institutional proficiency at transcaval access come at the expense of transaxillary proficiency? In this series, transaxillary and transcaval procedures were performed by experienced operators who proctor others in the techniques. The transaxillary complications were not attributable to the cases undergoing surgical rather than percutaneous access and were not confined to sites performing a low volume of transaxillary access. That said, while transaxillary mortality and bleeding were comparable to previous reports (TABLE 4), neurovascular complications appear higher than previous reports.
Third, does operator or institutional preference for one technique over another bias the results in favor of the first? Specifically, if operators prefer transcaval as a first nonfemoral option, are the patients undergoing transaxillary more likely to be ineligible for transcaval and therefore possibly at higher risk of transaxillary complications? In this study, 3 of 8 sites had stopped performing transaxillary access before the study period and contributed no transaxillary cases; an additional 3 of 8 sites contributed more transaxillary than transcaval cases arguing against a preferred route. Nevertheless such bias is possible and probably can only be mitigated by different study designs such as prospective randomization.
The incidence of transcaval bleeding in this study is lower than observed during early clinical experience in the transcaval IDE protocol(16). The reduced bleeding probably reflects three phenomena. First, operator technique and experience have improved, including universal protamine reversal of heparin anticoagulation before closure and liberal application of balloon aortic tamponade after implanting the closure device. Second, systematic CT surveillance of any TAVR access sites would likely reveal subclinical hematomas. The transcaval IDE trial uniquely among TAVR trials included systematic post-procedure access-site CT which we believe increased ascertainment of VARC-2 bleeding and vascular complications not otherwise clinically apparent. Third, in the past transcaval access may have been reserved for truly “no-option” patients with extreme co-morbidity, rather than employed as a routine alternative to transfemoral access. Such early “no-option” patients are expected to suffer more frequent complications including bleeding. The downward temporal trend in STS mortality-risk scores among transcaval-access patients reported in the literature and in this report (TABLE 4) supports this explanation. Fourth, transfusion strategies have become more restrictive (28). Commercialization of a non-permeable dedicated transcaval closure device (29) might further reduce bleeding. Finally, we observed a significantly lower incidence of our patient-oriented composite endpoint of “discharge directly to home without stroke or TIA” among patients undergoing either transcaval or transfemoral compared with transaxillary access. This is out-of-proportion to neurological events. One explanation may be that convalescence after transaxillary access may be more protracted than recognized.
Each access method has its relative advantages. Transfemoral artery access risks the fewest complications when appropriately selected, is ergonomically attractive, and confers the shortest length of stay. Transaxillary artery access, especially percutaneous, is more technically complex than transfemoral, but enjoys widespread operator and institutional experience and training opportunities and requires low materials costs when covered stents are not required. Recent technical refinements in transaxillary access include local anesthesia, which allows real-time monitoring and intervention to respond to neurovascular events; and radial-only accessory and bailout access (30) (12). Disadvantages include higher risk of stroke and bleeding compared with transfemoral, and risk of internal mammary artery bypass obstruction. By comparison, transcaval access has low transfemoral-like stroke rate, increased transaxillary-like bleeding rate, and transfemoral-like favorable operator ergonomics and radiation exposure. The main disadvantage of transcaval access is higher materials costs for the closure device and deflectable sheath, longer fluoroscopy time and contrast dose, and less-well-disseminated operator experience. Among nonfemoral access options, transcarotid access has advocates even though it is not fully percutaneous (31,32); participating sites contributed too few transcarotid (n=29) and transthoracic (n=34) TAVR procedures for meaningful comparison in this study. TVT Registry reports 586 transcaval and “other” access TAVR procedures in the years 2017–2019, and 5275 transaxillary procedures(22). We estimate our series represents approximately 30% of transcaval procedures and 1.5% of transaxillary procedures in the registry. We believe other centers can probably replicate the results of this study.
Limitations
Important limitations of this study include the non-random allocation of access routes, which were selected by the operators. Follow-up registries, such as the one used for this study, are subject to under-recognition and under-reporting of complications. Universal source-data verification and universal predischarge CT scanning of access sites would likely increase ascertainment of complications; this ascertainment bias likely applies equally across access routes. Allocation to transcaval or transaxillary access may reflect disproportionate referral bias of more risky cases to more experienced operators participating in this registry. Despite our attempts to compensate for baseline imbalances using doubly robust estimation (both propensity score and multivariate regression modeling), we cannot rule out unmeasured confounding. It is possible that patients selected for transaxillary access are indeed frailer and more prone to complications than those selected for transcaval access.
The observed disproportionate stroke after transaxillary access is mechanistically plausible and consistent with previous reports (Table 4). Whether surveillance for stroke/TIA is more intensive after transaxillary than other access routes is not known. We do not have data about stroke/TIA sidedness (localization to the site of transaxillary access), which might assist attribution of stroke/TIA to the TAVR procedure. The role (or feasibility in transaxillary access) of cerebral embolic protection is unclear given its sparse application in this study. Despite these limitations, transcaval access does not appear to have worse bleeding or vascular complications than transaxillary access; indeed, by some measures it appears superior.
CONCLUSIONS
Among experienced operators participating in this report, transcaval access conferred a lower risk of stroke, and a comparable rate of bleeding and vascular complications, than transaxillary access for TAVR. A prospective randomized trial comparing both approaches would be important to remove unrecognized bias in patient selection between the two groups. However, based on the neurovascular outcomes reported in our study, transcaval access should be considered an attractive percutaneous nonfemoral access option for TAVR.
PERSPECTIVES.
WHAT IS KNOWN?
Early reports of nonfemoral access suggested that transcaval access was associated with a higher incidence of bleeding and major vascular complications, and that transaxillary access may be associated with a higher incidence of stroke/TIA.
WHAT IS NEW?
Transcaval access had a lower rate of stroke/TIA than transaxillary access for TAVR, comparable bleeding and vascular complications, and higher rate of discharge directly to home without stroke/TIA.
WHAT IS NEXT?
Transcaval access techniques can be disseminated through training and proctorship. Dedicated closure devices may further reduce the incidence of bleeding after transcaval access.
ACKNOWLEDGEMENTS
We are grateful for help collecting data by L Roberts, E Charles, K Pitts at Emory; J May and T Calayo at Sentara; J Rowe and M Joseph at Carilion; E Schulz at Rochester General; TR Smith and M Brooks at Oklahoma Heart; L Hickman at Alexian Brothers; and to X Tian for graphics help.
Drs Babaliaros and Greenbaum have served as consultants for Edwards Lifesciences and Abbott Vascular; have an employer with research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific; and own equity interest in Transmural Systems.
Drs Lederman and Rogers are co-inventors on device patents, assigned to the National Institutes of Health, for closure of transcaval access ports.
Dr Rogers has served as proctor for Edwards Lifesciences and Medtronic.
Dr Rogers has served as consultant and physician proctor for: Edwards Lifesciences and Medtronic; Advisory Board: Medtronic; Equity Interest: Transmural Systems;
Dr Mahoney has served as proctor and consultant, and has received institutional research support from Edwards Lifesciences, Medtronic, and Abbott.
Dr Foerst has served as proctor for Edwards Lifesciences and Medtronic.
Dr Depta has served as consultant or advisory board member for Edwards Lifesciences, Boston Scientific, Abbott, V-Wave-Ltd, and WL Gore & Associates.
Dr Muhammad has served as proctor and consultant for Edwards Lifesciences and Medtronic.
Dr McCabe has served as consultant and has received honoraria from Boston Scientific, Cardiovascular Systems Inc, Edwards Lifesciences, and Medtronic.
Dr Pop is a consultant for Edwards Lifesciences and for Shockwave Medical.
Dr Khan has served as proctor for Edwards Lifesciences and Medtronic.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Disclosures/funding:
This work was supported by the Emory Structural Heart and Valve program intramural funds, and by National Institutes of Health Z01-HL006040.
ABBREVIATIONS AND ACRONYMS
- CHA2DS2-Vasc
Congestive heart failure, Hypertension, Age ≥75 (doubled), Diabetes mellitus, prior Stroke or transient ischemic attack (doubled), Vascular disease, Age 65–74, Female
- GBM
Generalized Boosted Modeling
- GMR
Geometric Mean Ratio
- IDE
Investigational device exemption
- NYHA
New York Heart Association heart failure classification
- STS-PROM
Society of Thoracic Surgeons’ Predicted Risk of 30-day Mortality
- TAVR
Transcatheter Aortic Valve Replacement
- TIA
Transient Ischemic Attach
- TVT
Transcatheter valve therapy (registry)
- VARC
Valve Academic Research Consortium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Svensson LG, Blackstone EH, Rajeswaran J et al. Comprehensive analysis of mortality among patients undergoing TAVR: results of the PARTNER trial. J Am Coll Cardiol 2014;64:158–68. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy FH, Spragan DD, Savino D et al. Outcomes, readmissions, and costs in transfemoral and alterative access transcatheter aortic valve replacement in the US Medicare population. J Thorac Cardiovasc Surg 2017;154:1224–1232 e1. [DOI] [PubMed] [Google Scholar]
- 3.Blackstone EH, Suri RM, Rajeswaran J et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation 2015;131:1989–2000. [DOI] [PubMed] [Google Scholar]
- 4.Huded CP, Tuzcu EM, Krishnaswamy A et al. Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke. JAMA : the journal of the American Medical Association 2019;321:2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal A, Kalra A, Kumar A et al. Outcomes of Combined Transcatheter Aortic Valve Replacement and Peripheral Vascular Intervention in the United States. JACC Cardiovasc Interv 2021;14:2572–2580. [DOI] [PubMed] [Google Scholar]
- 6.Schafer U, Deuschl F, Schofer N et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. International journal of cardiology 2017;232:247–254. [DOI] [PubMed] [Google Scholar]
- 7.Gleason TG, Schindler JT, Hagberg RC et al. Subclavian/Axillary Access for Self-Expanding Transcatheter Aortic Valve Replacement Renders Equivalent Outcomes as Transfemoral. Ann Thorac Surg 2018;105:477–483. [DOI] [PubMed] [Google Scholar]
- 8.Dahle TG, Kaneko T, McCabe JM. Outcomes Following Subclavian and Axillary Artery Access for Transcatheter Aortic Valve Replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc Interv 2019;12:662–669. [DOI] [PubMed] [Google Scholar]
- 9.Debry N, Trimech TR, Gandet T et al. Transaxillary compared with transcarotid access for TAVR: a propensity-matched comparison from a French multicentre registry. EuroIntervention 2020;16:842–849. [DOI] [PubMed] [Google Scholar]
- 10.Kirker E, Korngold E, Hodson RW et al. Transcarotid Versus Subclavian/Axillary Access for Transcatheter Aortic Valve Replacement With SAPIEN 3. Ann Thorac Surg 2020;110:1892–1897. [DOI] [PubMed] [Google Scholar]
- 11.Amer MR, Mosleh W, Joshi S et al. Comparative Outcomes of Transcarotid and Transsubclavian Transcatheter Aortic Valve Replacement. Ann Thorac Surg 2020;109:49–56. [DOI] [PubMed] [Google Scholar]
- 12.Ooms JF, Van Wiechen MP, Hokken TW et al. Simplified Trans-Axillary Aortic Valve Replacement Under Local Anesthesia - A Single-Center Early Experience. Cardiovascular revascularization medicine : including molecular interventions 2021;23:7–13. [DOI] [PubMed] [Google Scholar]
- 13.Lederman RJ, Chen MY, Rogers T et al. Planning transcaval access using CT for large transcatheter implants. JACC Cardiovascular imaging 2014;7:1167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederman RJ, Greenbaum AB, Rogers T, Khan JM, Fusari M, Chen MY. Anatomic Suitability for Transcaval Access Based on Computed Tomography. JACC Cardiovasc Interv 2017;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederman RJ, Babaliaros VC, Rogers T et al. The Fate of Transcaval Access Tracts: 12-Month Results of the Prospective NHLBI Transcaval Transcatheter Aortic Valve Replacement Study. JACC Cardiovasc Interv 2019;12:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum AB, Babaliaros VC, Chen MY et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J Am Coll Cardiol 2017;69:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa G, De Backer O, Pilgrim T et al. Feasibility and safety of transcaval transcatheter aortic valve implantation: a multicentre European registry. EuroIntervention 2020;15:e1319–e1324. [DOI] [PubMed] [Google Scholar]
- 18.Reardon MJ, Van Mieghem NM, Popma JJ et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. The New England journal of medicine 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 19.Popma JJ, Deeb GM, Yakubov SJ et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. The New England journal of medicine 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 20.Mack MJ, Leon MB, Thourani VH et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. The New England journal of medicine 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 21.Leon MB, Smith CR, Mack MJ et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. The New England journal of medicine 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 22.Carroll JD, Mack MJ, Vemulapalli S et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020;76:2492–2516. [DOI] [PubMed] [Google Scholar]
- 23.Jacques L, Syrek Jensen T, Schafer J, Fulton S, Schott L, Baldwin J. Coverage Decision Memorandum for Transcatheter Aortic Valve Replacement (TAVR) CAG-00430N. Baltimore, MD: Coverage and Analysis Group, Centers for Medicare and Medicaid Services, 2012. [Google Scholar]
- 24.Jensen TS, Chin J, Ashby L et al. Coverage Decision Memorandum for Transcatheter Aortic Valve Replacement (TAVR) CAG-00430R. Baltimore, MD. Coverage and Analysis Group, Centers for Medicare and Medicaid Services, 2019. [Google Scholar]
- 25.Long A, Mahoney P. Comparative Intermediate-Term Outcomes of Subclavian and Transcaval Access for Transcatheter Aortic Valve Replacement. J Invasive Cardiol 2020;32:463–469. [PubMed] [Google Scholar]
- 26.Genereux P, Piazza N, Alu MC et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol 2021;77:2717–2746. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 2013;32:3388–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carson JL, Triulzi DJ, Ness PM. Indications for and Adverse Effects of Red-Cell Transfusion. New England Journal of Medicine 2017;377:1261–1272. [DOI] [PubMed] [Google Scholar]
- 29.Rogers T, Greenbaum AB, Babaliaros VC et al. Dedicated Closure Device for Transcaval Access Closure: From Concept to First-in-Human Testing. JACC Cardiovasc Interv 2019;12:2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooms JF, Van Mieghem NM. Completely Percutaneous Transaxillary Aortic Valve Implantation Under Local Anesthesia: A Minimalist Alternative Access Approach. JACC Cardiovasc Interv 2019;12:e1–e2. [DOI] [PubMed] [Google Scholar]
- 31.Allen KB, Chhatriwalla AK, Saxon J et al. Transcarotid versus transthoracic access for transcatheter aortic valve replacement: A propensity-matched analysis. J Thorac Cardiovasc Surg 2020. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Monney P, Hullin R et al. Transcarotid Access Versus Transfemoral Access for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2021;8:687168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbash IM, Segev A, Berkovitch A et al. Clinical Outcome and Safety of Transcaval Access for Transcatheter Aortic Valve Replacement as Compared to Other Alternative Approaches. Front Cardiovasc Med 2021;8:731639. [DOI] [PMC free article] [PubMed] [Google Scholar]


