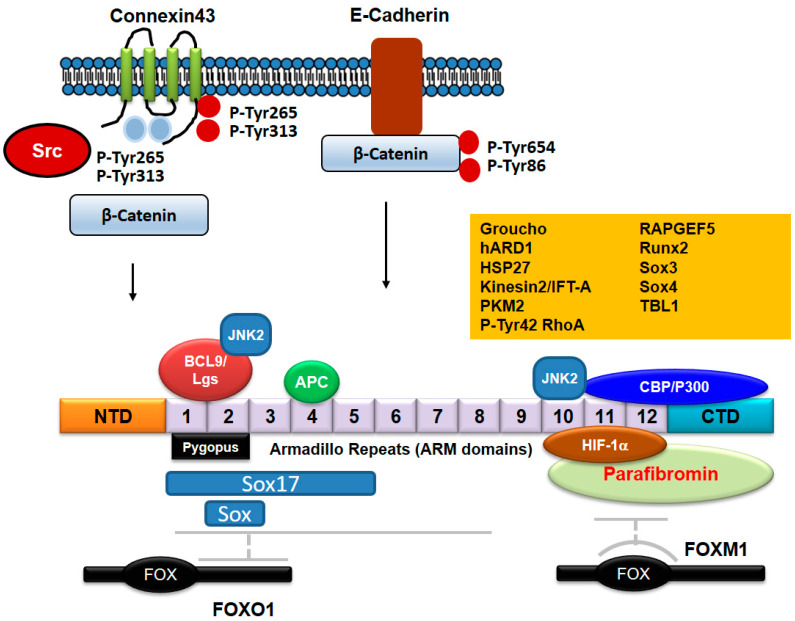
Figure 5.
Proteins binding to β-catenin. APC/Axin binds to ARM domains 3-7 of β-catenin. C-terminal domain of FOXO1 binds with ARMs 1-8 of β-catenin, whereas FOXM1 binds to ARMs 11-12 of β-catenin. Pygopus and BCL9/Lgs proteins bind to ARMs 1-2. CBP/P300 binds to C-terminal domain of β-catenin in addition to ARMs 11-12. Sox17 binds to β-catenin ARMs 1-5, but it is not clear that other Sox family proteins bind to which ARMs. JNK2 binds to both ARM domains 2 and 10. HIF-1α binds with β-catenin ARMs 10-12. Parafibromin binds to ARMs 10-12 including C-terminal domain. The exact regions of β-catenin domains binding with other proteins such as Groucho, hARD1, HSP27, Kinesin2/IFT-A, PKM2, p-Tyr42 RhoA, RAPGEF5, Runx2, and TBL1 still remain to be described. β-catenin binds to E-cadherin, but is released from E-cadherin by phosphorylation at Tyr86 and Tyr654 residues by Src. Connexin43 phosphorylated by Src at Tyr654 and Tyr86 residues reduces β-catenin interaction and connexin43 phosphorylated by Src at Tyr247 and Tyr265 residues reduces Src oncogenic activity.