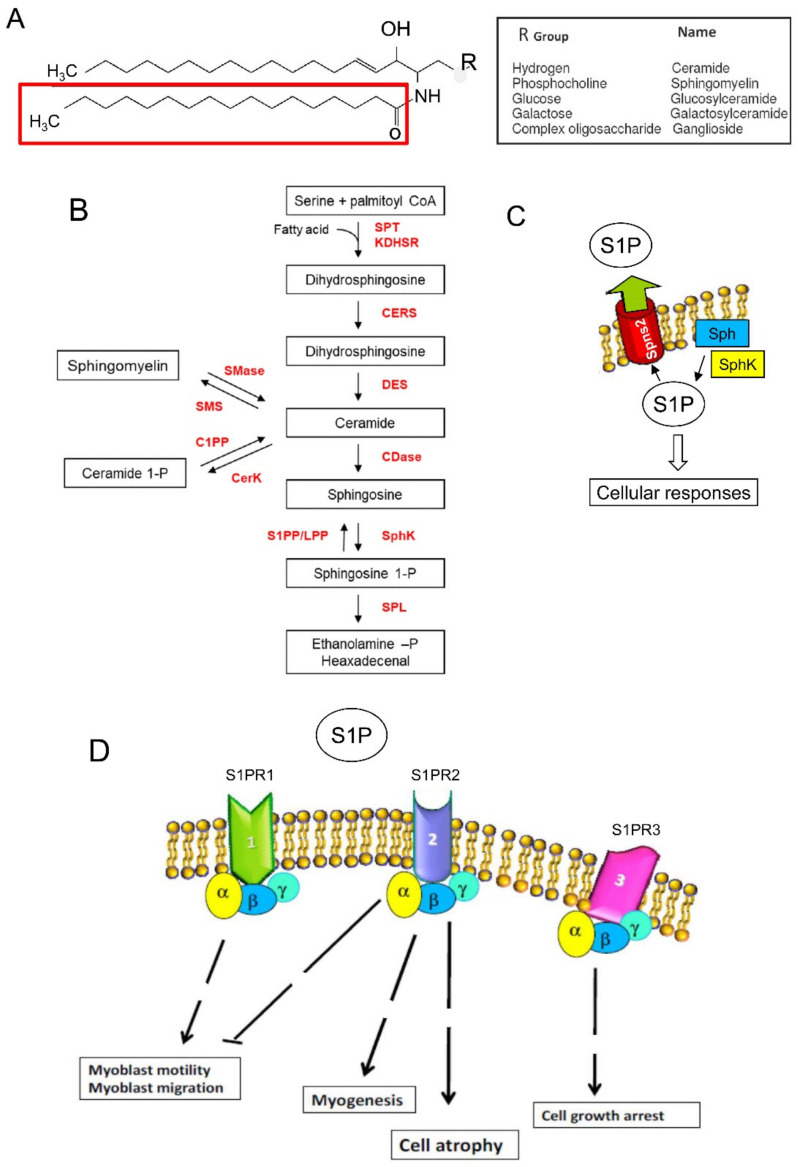
Figure 1.
Sphingolipid metabolism and sphingosine-1-phosphate (Sphingosine 1-P) signaling. (A) General sphingolipid structure. Sphingolipids are composed of a sphingosine backbone linked to a fatty acid. (B) Sphingomyelin cycle or de novo sphingolipid synthesis leading to ceramide involving serine palmitoyl transferase (SPT), 3-keto dihydrosphingosine reductase (KDHSR), ceramide synthase (CERS), and desaturase (DES). Ceramide is converted reversibly to sphingosine by ceramidase (CDase) or phosphorylated to ceramide-1-phosphate (Ceramide 1-P) by ceramide kinase (CerK) activity and dephosphorylated by ceramide-1P phosphatase (C1PP). S1P is synthesized from sphingosine by the sphingosine kinases (SphK) and irreversibly cleaved by S1P lyase (SPL), which generates hexadecenal and phosphoethanolamine (ethanolamine -P). S1P is also a substrate of specific S1P phosphatases (S1PP) or lipid phosphate phosphohydrolase (LPP). Sphingomyelin synthases (SMS) transfer a phosphorylcholine group from phosphatidylcholine to ceramide, generating diacylglycerol and sphingomyelin. Sphingomyelinases (SMase) catalyze the hydrolysis of sphingomyelin, leading to the generation of ceramide and phosphorylcholine. (C) S1P produced inside the cell can be transported in the intercellular space by an ATP-binding cassette transporter named spinster homolog 2 (Spns2). (D) As a ligand, S1P acts as autocrine and paracrine factors triggering specific signaling pathways by interacting with S1P specific heterotrimeric GTP binding protein-coupled receptors, named S1PR. Three among five subtypes of S1PRs, S1PR-1, -2, and -3, are expressed in skeletal muscle cells and regulate through different steps (broken lines) specific biological functions. The scheme exemplifies the main roles played by S1PR activation in skeletal muscle cells.