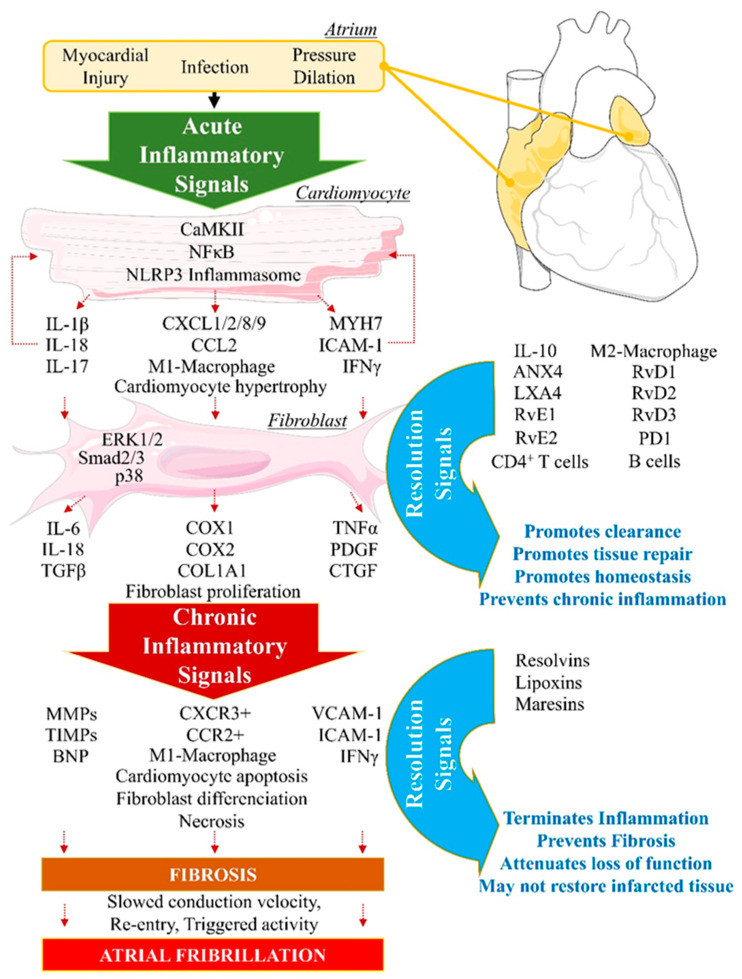
Figure 1.
Biomolecular orchestration of cellular events from cardiac insult to resolution opposed to persistent arrhythmogenic inflammation. Longstanding exposure of the atrium to myocardial injuries, infections or chronic pressure and dilation provokes the normal initiation of acute inflammation. In cardiomyocytes (CM), intracellular inflammatory response involves CamKII, NF-kB or NLRP3 inflammasome pathways activation, which contribute to CM deregulation of structural genes (Myh7), and secretion of proinflammatory cytokines including interleukins (IL-1β, IL-18) and chemokines (CXCL, CCL), leading to promotion of proinflammatory (M1)-macrophage infiltration. Proinflammatory signals contribute to the activation of cardiac fibroblasts (FB). FB activate additional pro-inflammatory signals (TGFβ, TNFα, PDGF) provoking FB differentiation into myo-FB, aiming to promote repair and wound healing, if the resolution signals are properly activated in response to inflammation initiation. Resolution mediators, including IL-10, LXA4, D- and E-series resolvins, contribute to terminate M1-macrophages infiltration, facilitate anti-inflammatory (M2)-macrophages polarization and phagocytosis, while activating CD4+ T cells and B cells efferocytosis, leading to homeostasis. When Resolution fails to occur, inflammation is perpetuated via FB and myoFB secretion of chronic-inflammation-promoting mediators (MMPs, IFNγ, CXCR3+, M1-macrophages) leading to CM necrosis, and loss of function. Resolution signals can be promoted to limit chronic inflammation-induced damages. If failed resolution mechanisms persist, the myocardium is exposed to the development of fibrosis, slowed conduction velocity, triggered activity, re-entry and increased susceptibility to arrhythmias, including atrial fibrillation.