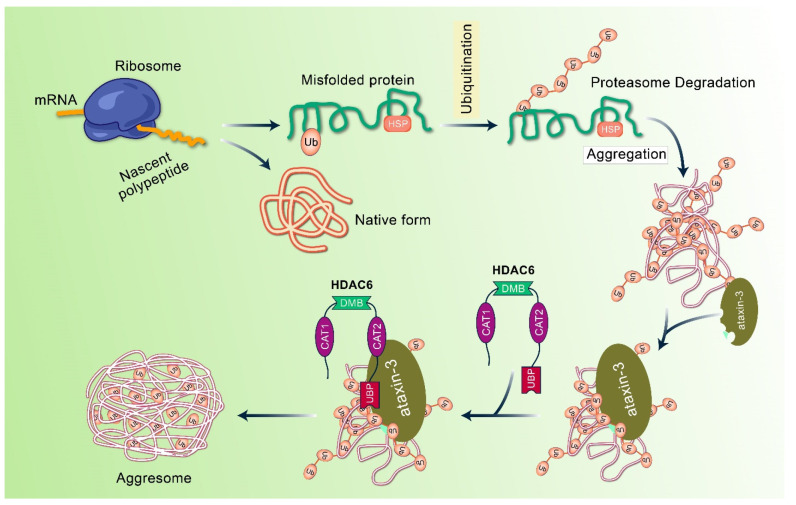
Figure 3.
Molecular mechanism of aggresome development and formation. Under normal conditions, misfolded and polyubiquitinated proteins are fragmented via the ubiquitin proteasomal system. When ubiquitin proteasomal system is altered or overhauled, misfolded polyubiquitin proteins accumulate and form to aggregate. In this case, ataxin-3-dexiquitinase interrelates and aggregates with polyubiquitinated proteins to form ubiquitin chain structure. In addition, HDAC6 binds these non-anchored C-terminal tails of ubiquitin to form aggregates and recruits them into the dynein motor complex.