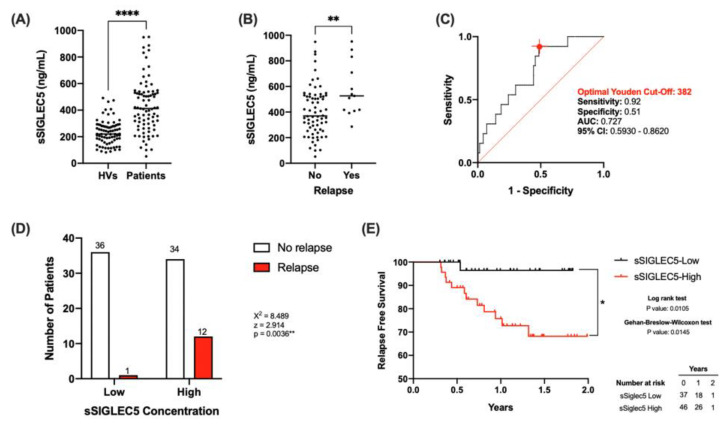
Figure 1.
Pre-operative soluble SIGLEC5 (sSIGLEC5) levels act as relapse predictors in lung cancer. (A) The soluble SIGLEC5 (sSIGLEC5) levels in plasma from healthy volunteers (HV, n = 67) and patients with lung cancer (n = 83) quantified by enzyme-linked immunosorbent assay are shown. The difference between groups was analysed by an unpaired Mann–Whitney U test; **** p < 0.0001. (B) sSIGLEC5 levels in patients who suffered relapse (n = 13) vs those who did not (n = 70) are shown. The difference between groups was analysed by an unpaired t test; ** p < 0.01. (C) Receiver-operating-characteristic (ROC) curve describing the predictive performance value of plasma sSIGLEC5 for relapse in patients with lung cancer (n = 83) (black line; area under the curve [AUC], 0.727 [95% CI 0.593–0.862]) is shown. Optimal cut-off, estimated by the Youden index for plasma sSIGLEC5 concentration, 382 ng/mL (red point shows optimal Youden cut-off specificity and sensitivity values). The ROC curve analysis was performed by Wilson/Brown test, with 95% confidence interval. (D) Chart shows number of patients who suffer relapse in the sSIGLEC5-High group (>382 ng/mL) compared with the sSIGLEC5-Low group (<382 ng/mL). Differences between groups were analysed with the chi-squared test, ** p = 0.0036. (E) Kaplan–Meier curve from surgery date to relapse or end of study date, according to plasma sSIGLEC5 is shown. The differences between relapse free survival rates associated with sSIGLEC5 were calculated by a log-rank (Mantel-Cox; * p = 0.0105) test and Gehan–Breslow–Wilcoxon (* p = 0.0145) test with 95% confidence interval.