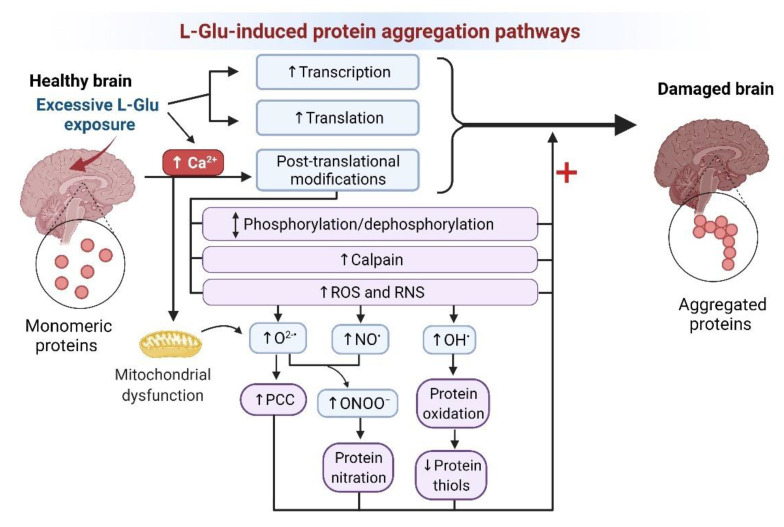
Figure 3.
L-Glu induction of neurotoxic protein aggregation mechanisms reported from in vitro and in vivo model studies. Excessive L-Glu exposure can potentially induce protein aggregation in neurons via an influence on transcription, translation, or protein post-translation modifications (PTMs). High L-Glu in the synaptic cleft induces excitotoxicity, resulting in high Ca2+ influx into neurons, and this triggers protein PTMs. PTMs arise via the activation of kinases and/or phosphatases affecting the levels of protein phosphorylation/dephosphorylation, calpain activation, or increased oxidative stress (ROS or RNS production). Excessive Ca2+ also causes mitochondrial dysfunction, resulting in ROS leakage, including O2−•, which causes protein oxidation and protein carbonylation. O2−• ions also interact with NO produced by nitric oxide synthase to produce reactive nitrogen species, such as ONOO−, which covalently modify proteins via protein nitration. L-Glu also increases the production of OH•, which can also cause protein oxidation and oxidation of protein thiols. Collectively, these PTMs could alter protein conformation and promote misfolding and protein aggregation. Abbreviations: Ca2+, calcium ions; NO•, nitric oxide; O2−•, superoxide; OH•, hydroxyl radical; ONOO−, peroxynitrite; PCC, protein carbonyl content; RNS, reactive nitrogen species; ROS, reactive oxygen species.