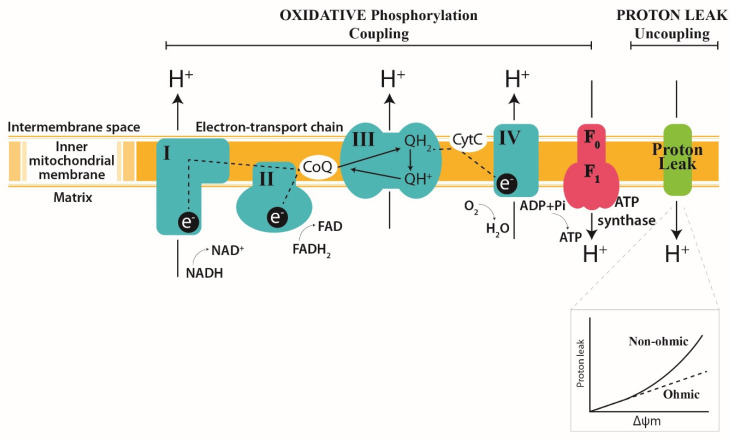
Figure 1.
Diagram of the inner mitochondrial membrane, showing three processes that underlie oxidative phosphorylation. (1) The electron transport chain; (2) the uncoupling of oxidation from phosphorylation by leaking H+ across the inner mitochondrial membrane (Leak); and (3) phosphorylation to generate ATP (ATP synthesis). Complex I and II oxidize NADH and FADH2 respectively, transferring the resulting electrons to ubiquinol, which carries electrons to Complex III. Complex III shunts the electrons across the intermembrane space to cytochrome c, which brings electrons to Complex IV. Complex IV then uses the electrons to reduce oxygen to water. The energy liberated by the flow of electrons is used by Complexes I, III and IV to pump protons (H+) out of the mitochondrial matrix into the intermembrane space. This proton gradient generates the mitochondrial membrane potential that is coupled to ATP synthesis by Complex V (ATP synthase) from ADP and inorganic phosphate (Pi). Light green and the insert show the proton leak’s kinetics. The respiration rate increases disproportionately with the membrane potential (non-ohmic proton conductance). NADH, reduced nicotinamide adenine dinucleotide; NAD+, oxidized nicotinamide adenine dinucleotide; FADH, reduced flavin adenine dinucleotide; FAD+, oxidized flavin adenine dinucleotide. The dotted line indicates the electron pathways. The green arrows represent substrate reactions. The black arrows show the proton circuit across the IMM. Complexes I-V are marked as I, II, III and IV in green; ATP synthase is marked in red. Q, ubiquinone; Cyt C, cytochrome c.