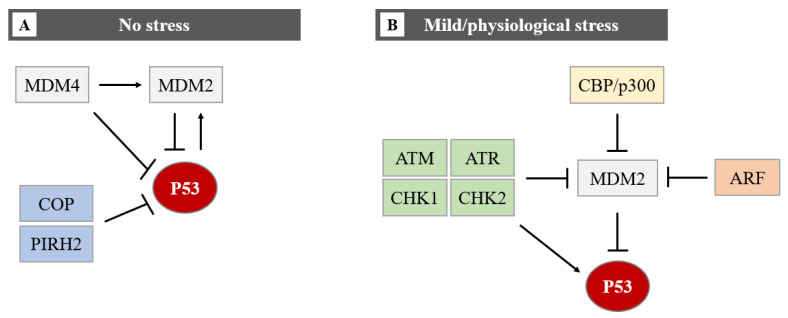
Figure 4.
The regulation of the P53 protein level in cells in no stress and mild/physiological stress. During no stress (A), E3-ubiquitin ligases (MDM2, COP, and PIRH2) inhibit P53 activity through polyubiquitination and proteasome degradation. The function of P53 and MDM2 is regulated by a negative feedback loop. MDM4 can bind directly to P53 by repressing P53 transcriptional activity, or it can promote MDM2-mediated degradation of P53. The MDM2-P53 association promotes the degradation of P53 because physiological degradation of P53 is ubiquitin-dependent and MDM2 acts as a P53-specific E3 ubiquitin ligase. In conclusion, as MDM2 expression is positively regulated by P53, MDM2 drives P53 proteasomal degradation, it becomes apparent that MDM2 and P53 form an autoregulatory loop. In this loop, MDM2 restrains P53 levels under normal physiological conditions. Under mild/physiological stress states (B), MDM2 levels are kept low by ATM, ATR, CHK1 and CHK2, ARF, and CBP/p300. The low level of MDM2 induces the expression of P53. In the presence of mild/physiological stress, the level of these proteins increases.