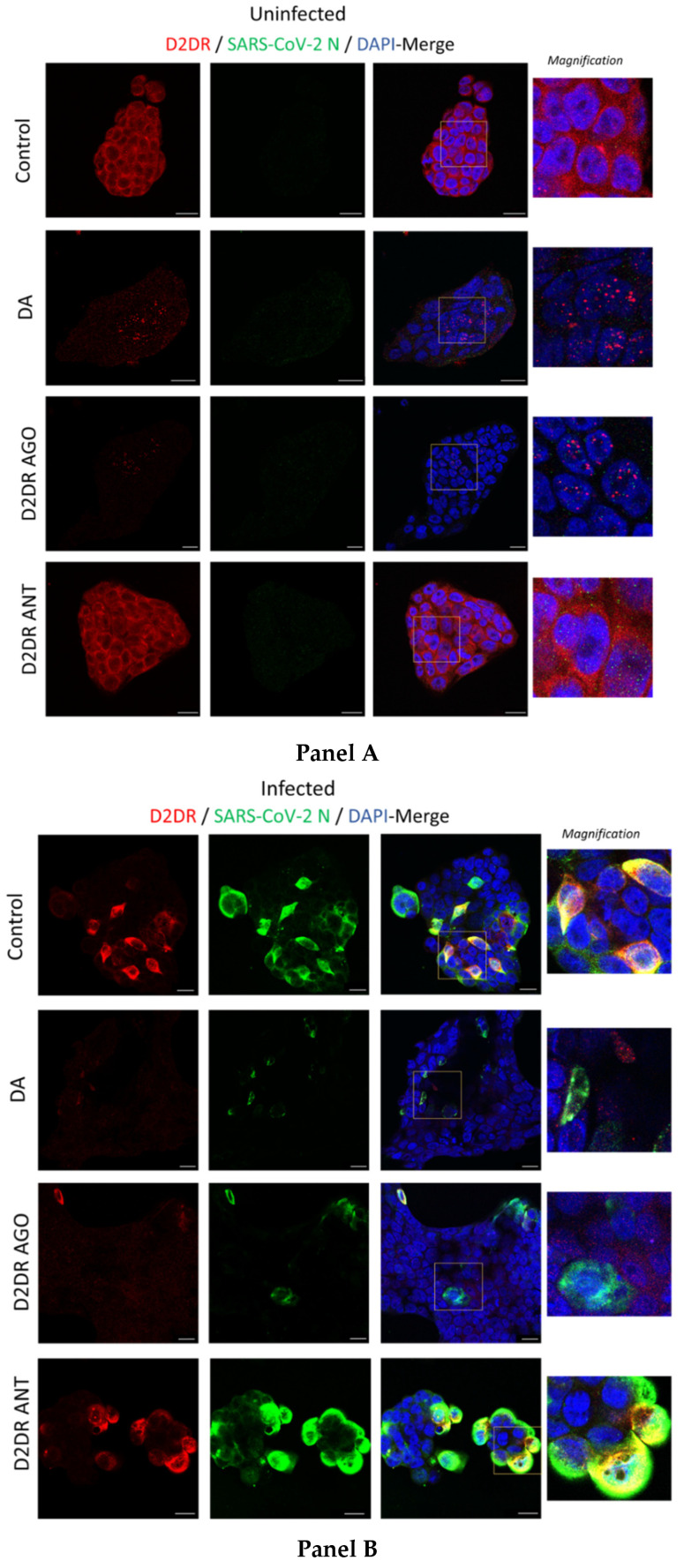
Figure 5.
Representative images of combined immunofluorescence for D2DR and SARS-CoV-2 N protein. The anti-replicative effects of dopamine (DA, 50 μM) and D2DR agonist quinpirole (10 μM) at 48 h post-infection are associated with downregulation of D2DRs in either Uninfected (Mock, Panel A) and SARS-CoV-2-Infected cells (Panel B). Compared with Uninfected Control cells (Panel A, Control), SARS-CoV-2 produces a dramatic clustering of D2DRs both at the plasma membrane and adjacent intracellular compartments, where an impressive co-localization with the SARS-CoV-2-N protein occurs (Panel B, Control). On the other hand, administration of DA, similar to the D2DR agonist, dramatically reduces D2DR staining leading to the formation of intracellular red puncta in both Uninfected (DA, and D2DR Ago, Panel A), and SARS-CoV-2-Infected (DA, and D2DR Ago, Panel B) cells. Such a scenario is not observed following D2DR antagonist administration in either Uninfected (Mock), or SARS-CoV-2-infected cells (D2DR Ant, Panel A and Panel B, respectively). In SARS-CoV-2 infected cells, the reduction in SARS-CoV-2-N protein staining that is produced by either DA or the D2DR agonist (Panel B) goes along with a dramatic reduction of D2DR immunostaining and a disruption of their co-localization. This is instead occluded by the administration of the D2DR antagonist, whereby the co-localization between D2DR and N-SARS-CoV-2 protein is magnified, as evident by the occurrence of both wide, overlapping areas and large intracellular puncta (D2DR Ant, Panel B). Cells were incubated with primary antibodies (Mouse anti-D2DR B-10: sc-5303, 1:200, Santa Cruz Biotechnology, Inc, Dallas, USA; Rabbit anti-N Nucleocapsid SARS-CoV-2 Antibody NR-53791, 1:1000, obtained from Bei Resources, NIAID, NIH), and with secondary antibodies [Goat anti-mouse Alexa Fluor 647 (ab150115), and Goat anti-rabbit Alexa Fluor 488 (ab150077), 1:500, abcam, Cambridge, UK]. Coverslips were mounted on Superfrost glass slides using a mounting medium with DAPI (Enzo Life Sciences, Milan, Italy). Confocal imaging was performed with a Leica TCS SP5 AOBS microscope system using a 40X/1.30 oil immersion objective (Leica Microsystems, Wetzlar, Germany). The images shown in the figures are representative results from independent experiments. Bars correspond to 20 μM.