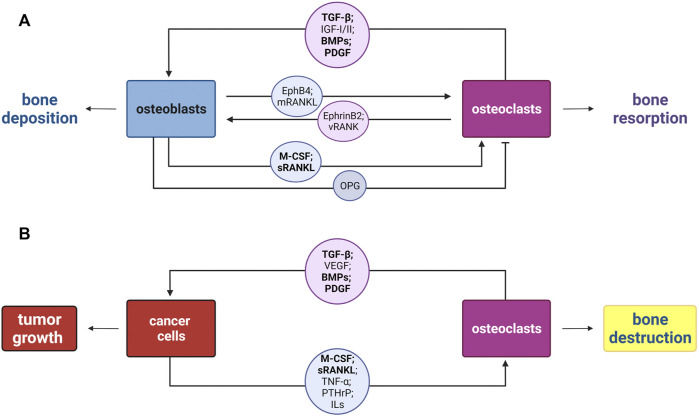
FIGURE 3.
Schematic representation of the osteoclast–osteoblast coupling in bone remodeling versus the vicious cycle in bone tumors. (A) New bone deposition occurs at bone resorption sites in each cycle of bone remodeling by the osteoblast–osteoclast coupling. Osteoblasts mediate both osteoclasts activation and inhibition, via secretion of M-CSF, soluble RANKL (sRANKL), and OPG. Instead, osteoclasts are able to mediate bone deposition by the release of matrix-embedded factors upon bone erosion (TGF-β, IGF-I/II, BMPs, and PDGF), through the secretion of extracellular vesicles carrying the receptor RANK (vRANK), and direct interaction through the membrane protein ephrinB2 with the osteblastic EphB4. (B) Disruption of the osteoblast–osteoclast coupling by the vicious cycle of bone. Tumor cells secrete soluble factors including M-CSF, sRANKL, TNF-α, PTHrP, and several interleukins (ILs), which act on osteoclast to enhance their bone destruction activity. Consequently, osteoclasts contribute to tumor growth and progression releasing TGF-β, pro-angiogenic factor VEGF, different BMPs, and PDGF. In bold are highlighted the molecules shared by the two cycles. M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor kappa B ligand; OPG, osteoprotegerin; TGF-β, transforming growth factor-β; IGF-I and IGF-II, insulin-like growth factor I and II; BMPs, bone morphogenetic proteins; PDGF, platelet-derived growth factor; RANK, receptor activator of nuclear factor kappa B; EphB4 and Eph receptor B4; TNF-α, tumor necrosis factor-α; PTHrP, parathyroid hormone-related protein; VEGF, vascular endothelial growth factor.