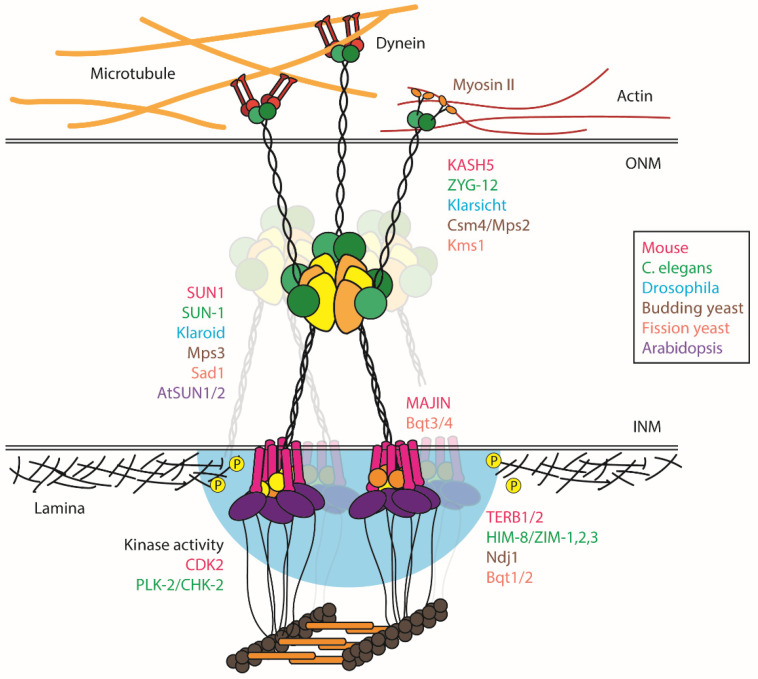
Figure 1.
Schematic representation of meiotic chromosome–NE–cytoskeletal complexes in diverse species. The SUN domain forms a trimer, and an adjacent extended α-helical domain forms trimeric coiled-coils, while KASH domain proteins dimerize. SUN domain trimers can dimerize and are proposed to interact with KASH domains from different dimers to create branched complexes, which may contribute to the clustering of chromosome attachment sites during meiosis. The nucleoplasmic domain of SUN proteins interact with chromosomal proteins, which often depends on the expression of meiosis-specific adapter proteins. Small proteins that span the inner nuclear membrane, including MAJIN in most metazoans and Bqt3/4 in fission yeast, are also essential for the coupling of chromosomes to the LINC complex and/or clustering of LINC complexes (translucent structures in the background). Cytosolic domains of KASH domain proteins often interact with dynein or microtubules; however, in budding yeast, they link telomeres to Myosin II on actin filaments. CDK2 and PLK-2/CHK-2 kinases are recruited to chromosome–LINC complex attachment sites in mice and C. elegans, respectively, and their activities (blue cloud) are required for the connection of telomeres/Pairing Centers to LINC complexes. In C. elegans, phosphorylation of the nuclear lamina by PLK-2 liquefies or disrupts the nuclear lamina to promote chromosome movements. In mice, CDK2 activity also limits promiscuous synapsis between nonhomologous chromosomes.