Abstract
Measuring immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 19 (COVID-19), can rely on antibodies, reactive T cells and other factors, with T-cell-mediated responses appearing to have greater sensitivity and longevity. Because each T cell carries an essentially unique nucleic acid sequence for its T-cell receptor (TCR), we can interrogate sequence data derived from DNA or RNA to assess aspects of the immune response. This review deals with the utility of bulk, rather than single-cell, sequencing of TCR repertoires, considering the importance of study design, in terms of cohort selection, laboratory methods and analysis. The advances in understanding SARS-CoV-2 immunity that have resulted from bulk TCR repertoire sequencing are also be discussed. The complexity of sequencing data obtained by bulk repertoire sequencing makes analysis challenging, but simple descriptive analyses, clonal analysis, searches for specific sequences associated with immune responses to SARS-CoV-2, motif-based analyses, and machine learning approaches have all been applied. TCR repertoire sequencing has demonstrated early expansion followed by contraction of SARS-CoV-2-specific clonotypes, during active infection. Maintenance of TCR repertoire diversity, including the maintenance of diversity of anti-SARS-CoV-2 response, predicts a favourable outcome. TCR repertoire narrowing in severe COVID-19 is most likely a consequence of COVID-19-associated lymphopenia. It has been possible to follow clonotypic sequences longitudinally, which has been particularly valuable for clonotypes known to be associated with SARS-CoV-2 peptide/MHC tetramer binding or with SARS-CoV-2 peptide-induced cytokine responses. Closely related clonotypes to these previously identified sequences have been shown to respond with similar kinetics during infection. A possible superantigen-like effect of the SARS-CoV-2 spike protein has been identified, by means of observing V-segment skewing in patients with severe COVID-19, together with structural modelling. Such a superantigen-like activity, which is apparently absent from other coronaviruses, may be the basis of multisystem inflammatory syndrome and cytokine storms in COVID-19. Bulk TCR repertoire sequencing has proven to be a useful and cost-effective approach to understanding interactions between SARS-CoV-2 and the human host, with the potential to inform the design of therapeutics and vaccines, as well as to provide invaluable pathogenetic and epidemiological insights.
Keywords: COVID-19, SARS-CoV-2, immune response, coronavirus, T-cell receptor repertoire, antibody, immunological memory, machine learning, diversity, immunoreceptor
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 19 (COVID-19), has resulted in devastating global morbidity and mortality [1]. Significant progress has been made into understanding the immunology of COVID-19 and developing various vaccines that offer high levels of initial protection. However, the longevity of both infection- and vaccination-induced COVID-19 immunity remains to be determined [2]. T cells play an important role in the pathogenesis and resolution of the disease and may provide long-lasting immunity [2]. This review explores the advances in our understanding of T-cell immunity to SARS-CoV-2 achieved through bulk T-cell receptor (TCR) repertoire analysis. To assist readers in interpreting the published studies, we first discuss the methodologies that can be applied, before presenting biological insights obtained from TCR repertoire analysis. Information obtained via such studies has implications for the design of vaccines and therapeutics, as well as for epidemiology and patient-risk stratification.
2. Overview of T-Cells in COVID-19
The relationship between T cells and clinical outcome in COVID-19 is complex and incompletely understood [2]. The peripheral blood T-cell count shows an inverse correlation with disease severity and a high CD8:CD4 ratio is observed in mild cases, with a decreased CD8:CD4 ratio correlating with severe disease. The poorer disease outcomes in patients over 65 years old are likely due to the decreased size (or diversity) of the naïve T-cell pool. This, coupled with COVID-19-induced lymphopenia, causes a delayed or uncoordinated adaptive immune response and suboptimal viral clearance [3]. Despite marked lymphopenia in severe disease, a marker of poor prognosis, the number of SARS-CoV-2 specific T cells in peripheral blood is higher than that of mild and convalescent individuals [4]. Flow cytometry and ELISA-based analysis of secreted cytokines (e.g., IFNγ) demonstrate that SARS-CoV-2 specific T cells in severe disease show a restricted cytokine profile, and high expression of inhibitory surface receptors and proliferation markers [4]. In severe cases of COVID-19, T-cell responses are either insufficient, inappropriate, or excessive [3,4,5].
2.1. Immunological Memory and Longevity
Understanding immunological memory is important for assessing the likelihood and severity of disease upon re-infection, as well as for epidemiological analysis and policy making. It is also a critical factor in determining how often booster vaccines should be offered to certain population subgroups, and clinically this may well be a potentially important role for T-cell immunity based tests in future. Evidence from SARS-CoV-1 suggests that T-cell immunity lasts for decades [6]. To date, antibody-based tests, such as lateral flow tests and ELISAs, have been used to infer immunity to SARS-CoV-2, but may not present a full picture. Neutralising antibody titres against the SARS-CoV-2 spike protein or receptor-binding domain decreased moderately over an 8-month follow-up period in 188 convalescent individuals. In contrast, 92% of individuals had detectable SARS-CoV-2-directed memory CD4+ T cells, as assessed by T-cell co-culture with SARS-CoV-2 peptides, followed by flow cytometric assessment of surface activation markers. The correlation between circulating antibody titres and T-cell immunity was incompletely understood [7]. Flow cytometry, using class I MHC tetramers and predicted optimal epitopes from SARS-CoV-2, demonstrated CD8+ specificity for SARS-CoV-2 in a higher percentage of subjects than antibodies, in a cohort of convalescent individuals, following mild or asymptomatic disease [8]. Furthermore, agammaglobulinaemic patients, who lack the ability to generate mature B cells or antibodies, can clear the SARS-CoV-2 infection without the need for mechanical ventilation, indicating that T cells may be sufficient to clear the infection with minimal symptomatic disease [3]. For some SARS-CoV-2 variants, such as the beta variant, against which Moderna (mRNA-1273) or Pfizer/BioNTech (BNT162b2) vaccine-induced antibody is relatively ineffective, as it is thought that a predominantly T-cell-mediated immune response still appears to confer immunity, at least decreasing disease severity and mortality [9,10,11]. It remains paramount to examine new variants that emerge for possible “T-cell escape” mutations.
2.2. T-Cell Cross-Reactivity
Assessing T-cell responses as evidence of previous SARS-CoV-2 infection is confounded by T-cell cross-reactivity with seasonal coronaviruses, with a study demonstrating cross-reactive T cells against the spike protein of SARS-CoV-2 in 28% of healthy unexposed (pre-pandemic) donor samples [8]. One benefit of this relatively high level of cross-reactivity in the unexposed population is the potential for some pre-existing immunity to SARS-CoV-2 infection [12].
2.3. Pathogenic Effects of T Cells
T cells are a sensitive and long-lasting marker of immunity in mild cases, but are potentially pathogenic in severe cases. Multisystem inflammatory syndrome (MIS) has pathogenesis similar to toxic shock due to the Staphylococcal antigens TSS toxin 1 and enterotoxin B. It can affect all major body systems in both children and adults, manifesting as persistent fever and hyperinflammation. Cytokine storms, occurring in some adults with COVID-19 are thought to have a similar aetiology [5].
2.4. T-Cell Tests in Current Clinical Use
Current clinical tests for T-cell immunity include enzyme-linked immunosorbent spot (ELIspot) and intracellular cytokine staining, both methods presenting T-cell storage, transport, and handling challenges. Newer technologies include Adaptive Biotechnologies sequencing-based analysis of the TCR repertoire, which shows higher sensitivity than commercially available serological tests without apparently being confounded by responses to other pathogens [13].
3. The TCR Repertoire
Each T cell has a TCR; the sequence, and to some extent, the antigen specificity of which is essentially unique to that cell and its clones. The combined set of TCR sequences from all T cells in an organism is termed the TCR repertoire. Its characteristics, including diversity, composition and dynamics, are important indicators of immune responses to specific antigens and are powerful tools for the diagnosis and prognosis of immune-related diseases.
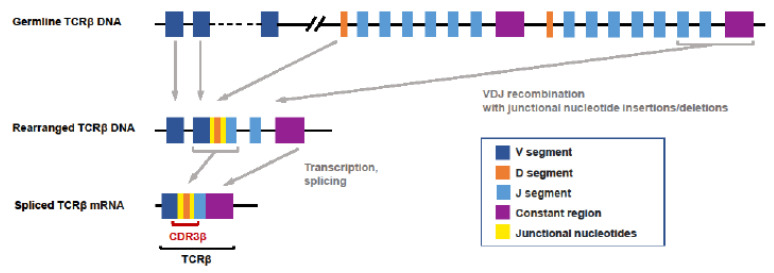
Similar sequences are present in cells with similar binding specificity, meaning that TCR repertoire sequencing can provide very useful insights into T-cell responses to a virus [14]. The mechanism by which unique TCR sequences are generated in each T cell is summarised in Figure 1. In brief, T cells, with receptors able to bind a very diverse array of antigens, contribute to the immunological protection of an individual. The genome would be unmanageably large if all unique DNA sequences required to encode these receptors were included in germline DNA [15,16]. TCRs are thus encoded in a combinatorial manner instead, with multiple separate DNA segments being brought together (Figure 1). This process occurs on two separate chains that are then paired, either as alpha-beta or gamma-delta, to present each T cell random, but unique, antigen specificity, which is not explicitly encoded by the genome. Subsequent positive and negative selections of T cells occur on the basis of the binding specificities of their receptors, removing in the thymus both those unlikely to bind antigen with appreciable affinity and those with autoimmune specificity, to leave a naïve mature T-cell repertoire. Because of the formation of junctions between germline-encoded gene segments at the variable positions, with potential template-independent insertion of bases, it can be appreciated that the genome will carry significant numbers of non-functional TCR rearrangements, for example, containing stop codons, due to shifts in the reading frame. Therefore, if sequencing is based on a DNA template, rather than RNA, it is important that non-functional TCR rearrangements are removed [17].
Figure 1.
V(D)J recombination determines T-cell receptor specificity. The TCR specificity of αβ T cells is determined by the unique V(D)J recombination events that occur during the development of each T cell. During this process, V, D and J gene segments are randomly selected and are spliced together on the β chain, while the α-chain rearrangement of the V–J gene segments occurs in a similar process. During this process, the random addition or deletion of nucleotides can occur at segment junctions. The complementarity-determining region 3 (CDR3) encoded by sequences located in the V(D)J junction has the greatest diversity and is what determines the antigen specificity of each TCR. TCRβ: T-cell receptor beta; CDR3β: the gene sequence encoding the complementarity-determining region 3 of the TCR beta chain.
Upon meeting a cognate antigen, a naïve T cell would be activated, leading to clonal expansion and differentiation into effector cells [15,16]. Expansion of multiple closely related clones might thus be expected in response to a given antigen.
3.1. Sample Cohort Building for TCR Repertoire Analysis
When comparing immunoreceptor repertoires between disease groups, it is critical that cohorts are as large as possible and ideally age- and gender- matched, as age, in particular, is known to affect the TCR repertoire composition [18]. Potential genetic and environmental confounders should also be considered.
3.2. Laboratory Methods in ‘Bulk’ TCR Repertoire Sequencing
Next-generation sequencing (NGS) has unleashed the ability to analyse the sequence of large numbers of TCRs in parallel. All of the TCR sequences in one sample are known as the TCR repertoire. Two types of TCR repertoire analysis dominate current research: “bulk” population sequencing and single-cell sequencing. The focus of this review is on “bulk” sequencing, which provides information on the frequency of single-chain usage, presenting a high-resolution view of diversity and of clonal relatedness, as whole populations of cells can be sequenced at a time. However, in order to assess chain pairings (either alpha-beta or gamma-delta), single-cell sequencing is required, which is typically more expensive and captures a smaller number of cells. As such, many of the analytical challenges and biological insights available to single-cell sequencing of the repertoire are different to those available to bulk sequencing, and are not covered in this review.
3.2.1. Substrate for Repertoire Sequencing
“Bulk” sequencing can be performed on DNA or RNA extracted from samples containing lymphocytes, most commonly blood, peripheral blood mononuclear cells (PBMCs) or pre-sorted lymphocytes and, less commonly, fresh frozen or formalin-fixed paraffin-embedded (FFPE) tissue. The advantages of blood or PBMC are non-invasive sample collection and availability of healthy control samples. DNA template has advantages over RNA, including greater stability and a 1:1 relationship between numbers of sequences and numbers of cells, rather than being confounded by transcript expression levels. For RNA, the number(s) of cells that had any given TCR sequence cannot be inferred. However, in our experience, a reasonably accurate assumption is that each unique complementarity-determining region 3 (CDR3) nucleic acid sequence comes from one cell only. In contrast, RNA has the advantage of only sequencing transcripts that are expressed, and thus likely to be functional (as explained in Figure 1), avoiding the need to screen out likely non-functional TCR sequences bioinformatically.
3.2.2. Methodological Considerations
Commonly used methods for producing TCR repertoire libraries are summarised in Table 1. Methods are generally amplification-based, using either 5′RACE (Rapid Amplification of cDNA Ends) or multiplex polymerase chain reaction (PCR). More rarely, methods are based on hybridisation capture [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Table 1). Most methods use the Illumina sequencing platform, although some utilise the Ion Torrent [42,43,44] or Roche 454 methodologies [45,46]. Caution must be taken when comparing repertoire data generated using different immunoreceptor library preparation methods, because of method-specific bias towards certain V and J segments, which is mainly a consequence of the use of method-specific PCR primers [47]. While each method has individual strengths [48], a gold-standard approach would improve the integration of results across different studies. Irrespective of library preparation method, incorporating unique molecular identifiers (UMIs) into nascent reads assists in distinguishing PCR duplicates from clonal sequences, as well as avoiding artefacts arising from PCR bias or PCR/sequencing errors [32,47], although UMI incorporation can be challenging when working with multiplex PCR methods.
Table 1.
Principles of laboratory methods for TCR repertoire sequencing.
| Method | DNA/RNA | Principles | Advantages | Disadvantages | Examples of Manufacturers |
|---|---|---|---|---|---|
| 5′RACE | RNA |
|
|
|
iRepertoire, Clontech/Takara, MiLaboratories |
| Multiplex PCR | DNA, RNA |
|
|
|
Adaptive Biotechnologies (Immunoseq); Invivoscribe (Lymphotrack) |
| Hybridisation capture | DNA, RNA |
|
|
|
Bespoke approaches |
3.3. Analysis of “Bulk” TCR Repertoire Sequencing
The CDR3 sequences are of primary interest in classification analyses because CDR3 regions are the most diverse and directly interact with antigens [49]. Pre-processing of sequencing data before alignment may be required if UMIs are incorporated. Accordingly, sequencing reads in fastq or fasta format are aligned to a reference database of V(D)J segments (e.g., IMGT (www.imgt.org), GenBank (https://www.ncbi.nlm.nih.gov/nucleotide/)) and clonotypes are assigned on the basis of V-, D- and J-segment usage and CDR3 lengths and sequences [50], using tools, such as MiXCR [51], IgBLAST [52] and IMGT/HighV-QUEST [53], with these different bioinformatic methods producing substantially different outputs [54]. While bespoke computational approaches are used by some, various immune repertoire analysis platforms are available. For example, VDJtools [55] or VisTCR [56] can be used to understand the immune repertoire by calculation and visualisation of summary statistics, pertaining to some of the parameters described in Table 2. A similar platform, ARResT [57], combines IMGT/HighV-QUEST with the analysis and visualisation of the resulting immune repertoire data. The adaptive immune receptor repertoire community (AIRR-C) is one of a number of repositories for COVID-19 TCR repertoire data [58].
Table 2.
Diversity-based methods of TCR repertoire analysis.
| Analytical Approach | Principles/Interpretation |
|---|---|
| CDR3 length profiles [59] |
|
| VDJ usage |
|
| Clonal abundance |
|
| Clonal frequency |
|
| Richness [62] |
|
| D50 diversity [64] |
|
| Simpson diversity [65,66,67] |
|
| Shannon diversity [61,67,69,70,71] |
|
| Hill’s diversity (Hill’s evenness) [73] |
|
| Pielou’s evenness index [64] |
|
| Parametric methods [74] |
|
3.3.1. Principles of Analysis of CDR3 Sequences
Multiple different analyses are possible (Table 2 and Table 3), including simple descriptive analyses (CDR3 length, V(D)J-segment usage, amino acid proportions), identification of clonotypes and their neighbours, more complex mathematical analyses of diversity, richness and evenness (Table 2), identification of specific motifs (Table 3), and machine learning methods. However, all these methods are subject to amplification bias, the type of starting material (DNA or RNA) and the quality and number of cells in the starting material.
Table 3.
TCR clustering methods. indicates that the TCR clustering method uses the feature to define a cluster.
indicates that the TCR clustering method uses the feature to define a cluster.
| Method | Features | |||||||
|---|---|---|---|---|---|---|---|---|
| V(D)J Alignment |
CDR3s | Short Motifs | Physio-Chemical Properties | Amino Acids | Nucleotides | Frequency | Enrichment | |
| GIANA [82] |
 (V Only) (V Only) |
 |
 |
 |
||||
| ALICE [83] |  |
 |
 |
 |
||||
| clusTCR [84] |  |
 |
||||||
| GLIPH2 [85] |  |
 |
 |
 |
 |
|||
| iSMART [86] |  |
 |
 |
 |
 |
|||
| TCRdist [87] |
 (CDR1 and 2) (CDR1 and 2) |
 |
 |
|||||
| TCRNET [83] |
 (V and J) (V and J) |
 |
 |
 (control samples required) (control samples required) |
||||
| ImmunoMap [88] |  |
 |
 |
|||||
| MiXCR [51] |
 (V and J) (V and J) |
 |
 |
|||||
3.3.2. Clonotypic Analysis
Simple analysis, such as CDR3 length profiles and V(D)J usages (Table 2), can be very effective at identifying clonality in samples. However, individual sequences are not considered and fine details may be obscured within these statistics. Clonotypic frequencies [50] provide a more in-depth perspective, and may vary between different patient groups, or between patients with a particular condition and controls. Any highly abundant receptors are assumed to be part of an active immune response whilst receptors that are observed in samples from multiple individuals with similar clinical conditions are thought to be capable of binding to a shared antigen [17]. Clonotypic analysis may focus on identifying known COVID-19-associated TCR sequences [49]. Analysis restricted only to clonotypically identical sequences may be too strict, particularly when considering the repertoires of different patients [17], meaning that motif-based analysis may provide more biologically meaningful information.
3.3.3. Diversity Profiling and Related Analyses
TCR diversity is a measure of the numbers of different CDR3 clonotypes in a sample and can be measured in multiple ways (Table 2) [17,49,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. Diversity measures adapted from ecology have commonly been used to characterise the TCR repertoire, particularly Shannon diversity, Simpson diversity and Hill diversity [67,74,76]. In the context of repertoire profiling, they describe TCR clonotype abundance, richness (number of unique clones) and evenness (the degree to which different clonotypes are equally represented in the sample). The different measures place different levels of importance on clonal characteristics, for instance, Simpson diversity is more sensitive to clonal dominance, whereas Shannon diversity is more sensitive to rare clonotypes. Although Pielou’s evenness index provides a more thorough description of repertoire structure than Simpson or Shannon diversities, none of these measures looks at individual sequences, but rather aim to characterise the repertoire as a whole.
3.3.4. Analyses Based on Sequence or Motif Identification
While analysing TCR metrics, such as the abundance of T cells, unique CDR3 sequences, or entropy, can provide an assessment of the diversity of the repertoire or its level of clonal expansion, these metrics are sequence agnostic. They are unable to assess the antigen-specific nature of the repertoire, and, furthermore, cannot identify antigenic associations with clinical outcomes in datasets. Clustering methods aim to group together TCR sequences that are either clonally related, having very similar CDR3s, or which likely bind the same antigen, having conserved motifs or similar physiochemical properties (Table 3). In identifying the clusters related to a particular disease, some methods use the cluster frequency or over-representation relative to a control group. It should be noted that the identification of such motifs is computationally demanding due to the vast number of combinatorial possibilities, and the methods presented in Table 3 often have a speed trade-off as the complexity of the algorithm and the patterns identified increase. Any identified motifs still require some form of validation, either in an independent test set or experimentally, before their real clinical utility can be exploited. Notwithstanding, multiple approaches have been taken to predict the likely antigens bound, including NetTCR, TCRex and MIRA, and databases, such as VDJdb and McPAS-TCR have been created to facilitate the sharing of known antigen specificities of TCR sequences [77,78,79,80,81].
3.3.5. Machine Learning to Predict Diagnosis, Exposure to Infection or Outcome of Infection
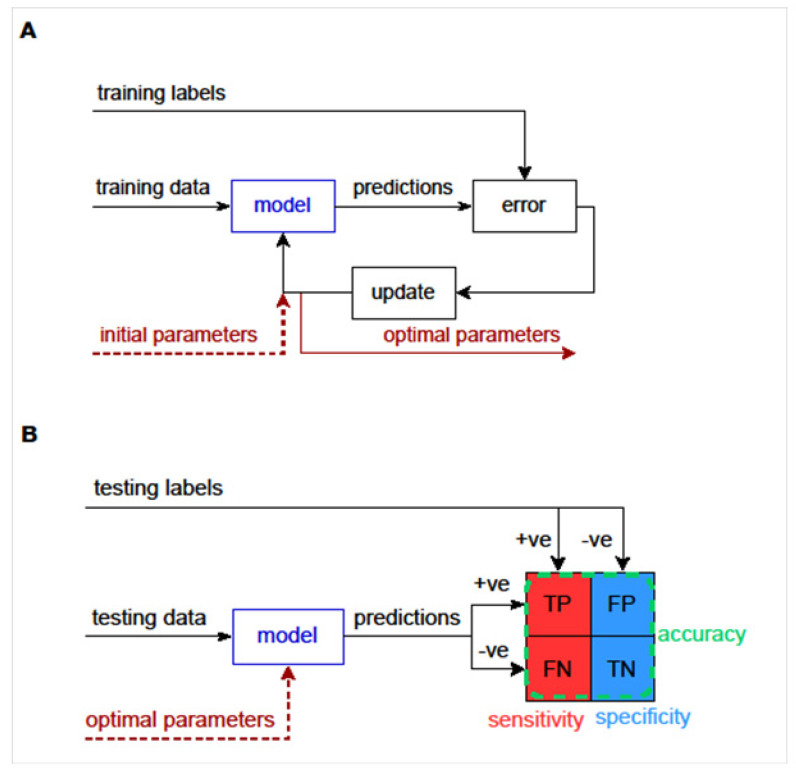
Machine learning algorithms (artificial intelligence, AI), which can be trained using examples, may be applied to produce TCR repertoire-based diagnostic models that can classify samples by diagnosis (e.g., COVID-19 unexposed versus previously COVID-19 infected). Such models can be built without the knowledge of the specificities of TCRs driving the classification and are typically supervised, in that they are provided with data from samples with a known label. Training can involve the selection of model parameters that produce the most accurate classification (Figure 2A). Such classification may be performed on the basis of closely related full-length CDR3 sequences [89], permitting 1 to 2 amino acid substitutions, often with weightings for the relatedness of substituted amino acids [90]. Alternatively, grouping may be performed on the basis of shorter motifs that form part of CDR3 sequences [14]. Testing then involves applying these optimal model parameters to a new set of testing data, in order to determine the classification accuracy (Figure 2B).
Figure 2.
Overview of machine learning approaches. (A) Training data and training labels are used to train the model by obtaining its optimal parameters. The model with initial parameters makes predictions for the training data. These predictions are compared to the training labels and the error between them is calculated. The model parameters are updated to correct for the error. These steps continue until the error cannot be made smaller and the model is trained. (B) A trained model should be tested with a separate set of data and labels called the testing data. Positive or negative predictions are made for the testing data using the trained model with its optimal parameters. Each prediction is compared to the positive or negative testing label and categorised as a true positive (TP), false positive (FP), true negative (TN) or false negative (FN). The model’s sensitivity can be estimated as TP/(TP + FN), specificity as TN/(TN + FP) and accuracy as (TP + TN)/(TP + FP + TN + FN).
To present an example, we successfully used clustering combined with a supervised training approach [17], to construct a classifier to separate samples donated by COVID-19 convalescent individuals from COVID-19-naïve individuals [14]. In our approach, CDR3 sequences were broken into contiguous amino acid sequences of length k (kmers), so that CDR3s that were non-identical, but shared a short motif, could be considered similar. The kmer length that provided the optimal classification was identified during the training stage [14]. Although this model for previous COVID-19 infection performed well in leave-one-out cross-validation, it has not been tested on any independent test sets analysed with the same laboratory methodology, due to small cohort sizes. The generalisability of this classifier between datasets thus remains unknown. A CDR3-based machine learning method, i-CAT, was also recently described to be able to separate TCR repertoires from individuals post-SARS-CoV-2 infection from unexposed individuals. However, the sample cohorts were exceptionally small, meaning that overfitting may have occurred, producing a falsely high accuracy [89]. The machine learning algorithm, DeepTCR, is a multiple-instance deep learning repertoire classifier assessing a combination of CDR3 sequence and V/D/J gene-segment usage [91], which has been used successfully to predict patients with severe versus milder SARS-CoV-2 infection from their repertoire sequencing [92]. However, it did not generalise between two separate cohorts, likely due to geographical and demographic differences, although overfitting could not be excluded. DeepTCR includes a convolutional neural network and is a platform for deep learning that can be applied at the level of individual TCR sequences or the whole TCR repertoire. It can learn patterns in the data that may be used for both descriptive and classification/predictive purposes [91]. Most Machine learning approaches are currently limited by the sample size. Although combining data from multiple sources could improve the robustness of these models with an enlarged training dataset, it is important to keep in mind the limitations of doing this due to different TCR sequencing methods, library preparation and target enrichment. However, datasets are increasing in size and, therefore, we expect machine learning-based classification models to increase in their utilities.
3.3.6. Machine Learning to Identify New Antigen-Specific Sequences
Machine learning has previously been used for the identification of novel sequences in TCR repertoires [93] and this has also been attempted for SARS-CoV-2. For example, using DeepTCR, 25 sequences most predictive of severe COVID-19 infection were identified. Multiplex Identification of T-cell Receptor Antigen Specificity (MIRA) was applied to these sequences and SARS-CoV-2 antigen specificity was predicted and shown to differ (a) between CD4 and CD8 T cells and (b) between individuals with mild and severe disease. As a consequence of this approach, it was possible to construct an epitope-specific classifier to predict whether patients had mild or severe disease [92].
4. Biological Insights into COVID-19 from T-Cell Receptor Analysis
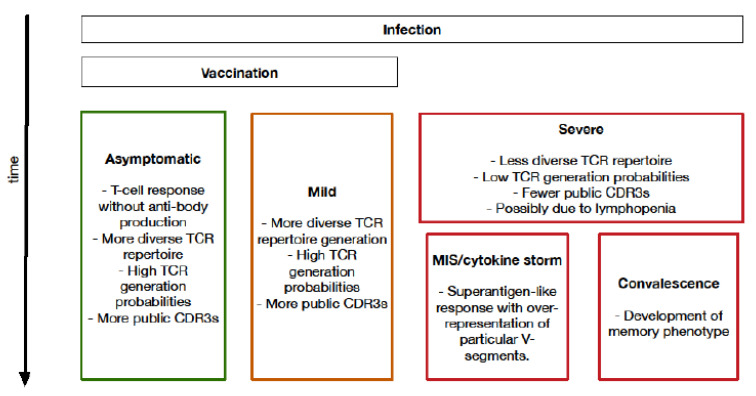
A broad range of biological insights have been gained from bulk TCR repertoire sequencing in COVID-19. Apart from the specific TCR sequences identified, which are contained in the various publications we cite, an overview of many of the biological findings can be observed in Figure 3 and summarised in Table 4. These publications were identified through a PuBMed search for papers containing the terms ‘T-cell receptor [TCR] sequencing’ or ‘T-cell receptor [TCR] repertoire’, and ‘COVID-19’ or ‘SARS-CoV-2’ in their title or abstract. Papers were then manually screened for their use of bulk TCR sequencing. Whilst these papers all represent interesting results, many of these studies are limited in their sample sizes, availability of healthy controls or pre-pandemic samples and lack of HLA typing.
Figure 3.
Schematic overview of insights into TCR repertoire, observed over time after infection, obtained by bulk TCR repertoire sequencing. MIS: Multisystem Inflammatory Syndrome.
Table 4.
Biological insights into COVID-19 from bulk TCR repertoire sequencing.
| First Author | Number of Samples |
Cells | DNA/RNA | Loci | Key Points |
|---|---|---|---|---|---|
| Chang [97] |
|
PBMCs | RNA | TRB |
|
| Cheng [5] |
|
PBMCs | DNA | TRB |
|
| Minervina [99] |
|
CD4+ and CD8+ T cells | RNA | TRB TRA |
|
| Niu [96] |
|
PBMCs | RNA | TRB TRA TRG TRD |
|
| Rajeh [89] |
|
PBMCs | RNA | TRB TRA |
|
| Schultheiss [95] |
|
PBMCs | DNA | TRB |
|
| Shomuradova [94] |
|
CD4+ and CD8+ T cells | RNA | TRB TRA |
|
| Shoukat [14] |
|
PBMCs | DNA | TRB |
|
| Sidhom [92] |
|
PBMCs | DNA | TRB |
|
| Swanson [99] |
|
PBMCs | DNA | TRB |
|
| Wang [100] |
|
CD4+ and CD8+ T cells | RNA | TRB TRA |
|
| Hu [101] |
|
PBMCs | RNA | TRB |
|
| Simnica [102] |
|
PBMCs Brain-derived T cells; |
DNA | TRB |
|
| Shimizu [103] |
|
CD8+ T cells | DNA | TRB TRA |
|
| Li [104] |
|
PBMCs | RNA | TRB |
|
4.1. Relative Contributions of T Cells and B Cells to SARS-CoV-2 Immunity
Shomuradova and colleagues showed that healthy donors during the pandemic had increased numbers of SARS-CoV-2-specific T cells, but not antibody response, likely indicating either prior asymptomatic SARS-CoV-2 infection or the presence of pre-existing cross-reactive T cells that represented a response to previous infection with a related virus [94]. Furthermore, in the same study, some convalescent patients had anti-SARS-CoV-2 TCRs, but no detectable antibody response after a certain period post-infection. Our own analysis of the convalescent cohort that had had mild infection in Schultheiss and colleagues’ study [95] showed an ability to predict SARS-CoV-2-immunity from TCR, but not BCR, repertoire data [14]. This indicates that there are fewer common features of BCR than TCR repertoire data between previously SARS-CoV-2 infected individuals, which may be because a SARS-CoV-2-specific B-cell response was limited in strength and duration or absent in a proportion of the individuals studied.
4.2. Association between Higher Repertoire Diversity and Improved Outcomes
Multiple studies have shown that, in mild infections with SARS-CoV-2, the TCR repertoire remains relatively diverse, with high generation probability (i.e., broadly predictable from germline rearrangement patterns) TCR sequences persisting. This indicates that the repertoire does not simply consist of T cells responding to a specific antigen. Furthermore, the frequencies of specific clonotypes, even those that are SARS-CoV-2-specific, are not particularly high, in contrast to findings in severe COVID-19, in which there are smaller numbers of more frequent SARS-CoV-2-specific sequences. Notwithstanding, a broad range of SARS-CoV-2-specific sequences are seen in mild disease, with many CDR3 sequences shared between multiple individuals, i.e., public CDR3 sequences [92,94,95,96,97]. One caveat is the fact that younger age may confound this observation. This is because younger age is associated both with milder COVID-19 disease and with broader TCR repertoires than in older people [18]. In both mild and severe disease, the TCR repertoire in peripheral blood increases in diversity during convalescence. Asymptomatic infection is thought to follow a similar course, in terms of TCR repertoire profiles, to mild disease [94].
4.3. Kinetics of CD4 and CD8 T-Cell Responses
CD4+ and CD8+ T-cell clonotypes both undergo transient clonal expansion after infection, with similar kinetics, with clonal contraction after day 15 and the majority acquiring effector memory phenotypes by day 30. A study of only 2 patients showed a separate episode of T-cell expansion on days 15–37 after the infection [98]. While this may have been due to priming of more T cells by antigen-specific B cells, migration of SARS-CoV-2-specific T cells from lymphoid organs or bystander activation of non-SARS-CoV-2 specific T cells, the possibility that this was due to triggering by another infection cannot be excluded, and so this second wave of CD4/CD8 T-cell expansion requires corroboration in other larger studies.
4.4. Importance of Specific V-, D- and J-Segment Usage
Few studies have found a very strong association between particular V-, D- and J-segment usage and prior exposure to SARS-CoV-2. However, in patients with a severe/hyperinflammatory COVID-19 clinical picture, four TCR Vβ gene segments (TRBV5-6, TRBV14, TRBV13 and TRBV24-1) were found to be overrepresented with little Jβ gene-segment skewing [5], suggesting a selective pressure preferentially acting on V-segment distribution. The same paper also used computational models and demonstrated that the spike protein of SARS-CoV-2, in contrast to other coronaviruses, exhibits a high-affinity motif for binding TCRs and may form a ternary complex with MHC-II, permitting it to behave similar to a superantigen, such as staphylococcal enterotoxin B. This provides a possible explanation for SARS-CoV-2 causing a cytokine storm in some adults and multisystem inflammatory syndrome in children and some adults.
4.5. Importance of SARS-CoV-2 Specific TCR Sequences and Motifs
TCR specificity was predicted in some of the datasets shown in Table 4 in one of two ways. One method is to undertake a functional assay, such as T-cell stimulation assays with subsequent flow cytometric analysis of cell surface phenotype and assaying T cells for their ability to bind to a fluorescently labelled MHC tetramer refolded with a selected SARS-CoV-2 peptide antigen [94,95]. The TCR repertoires of these likely SARS-CoV-2-specific T cells can thus be sequenced and analysed. The second approach is prediction by analogy to TCR sequences, the specificity of which is already known. The Multiplex Identification of the T-cell Receptor Antigen Specificity (MIRA) platform [80] was used for this purpose in several studies [92,98,99]. Between these two approaches, numerous potentially SARS-CoV-2-specific TCR sequences were identified.
4.6. Vaccine-Induced T-Cell Responses in Comparison to Responses to Native Infection
TCR repertoire responses to the AstraZeneca AZD1222 COVID-19 vaccine show similar changes to mild infection [99]. A total of 233 participants were vaccinated with AZD1222 or the MenACWY vaccine as a control, with doses approximately 4 or 12 weeks apart. Post-vaccination, participants had a significant increase in the fraction of total peripheral blood T cells and fraction of unique TCRs that were spike protein-specific 28 days after the second vaccine dose, with a similar depth and the breadth of the responses regardless of the dosing schedule. The breadth and depth increases were comparable to COVID-19 convalescent patients and no increase in TCR breadth of non-spike protein-specific SARS-CoV-2 TCRs was observed. As seen in SARS-CoV-2 infection [92], post-vaccination CD4 T-cell responses could be mapped to a broad range of parts of the spike protein, but CD8 responses were more restricted, most likely due to HLA restriction [99]. Peer-reviewed publications containing TCR repertoire data for other vaccines are awaited.
5. Areas for Further Research
Significant inroads have been made into understanding COVID-19 by means of analysis of TCR repertoire data, but much remains to be done. Further integration with single-cell data, in which chain pairing is known, will provide new insights, as will the ability of additional binding or functional studies to delineate new SARS-CoV-2-specific TCR sequences. Detailed mapping of particular TCR sequences to SARS-CoV-2 provides a tool kit for understanding the likely impact of new SARS-CoV-2 mutations upon largely vaccinated populations. While decreased TCR diversity acts as a useful biomarker to predict poorer prognosis, further identification of specific TCR sequences that are associated with unfavourable outcomes is desirable. In the context of vaccination, it is important to understand the risk of COVID-19 infection and/or severe disease post-vaccination as a function of the presence of specific TCR sequences. This requires large longitudinal studies of TCR repertoires in vaccinated individuals.
6. Conclusions
T cells form the backbone of the immune system and it is, therefore, of little surprise that they play such critical roles in determining the outcomes of infection with COVID-19. T cells carry natural “barcode” sequences, by virtue of their TCR variable-region sequences, particularly the CDR3 component. This gift of nature has provided the opportunity to study T cells in great detail and to begin answering key questions about the course and longevity of infection or vaccine-induced immunity to SARS-CoV-2.
Author Contributions
H.K., S.E., T.D., E.C., A.K., A.E., K.N.L.H., S.W., A.F. (Andrew Foers), A.F. (Anna Fowler) and E.J.S. contributed to the original writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
E.S. and A. Fowler (inventors) of GB Patent Application No: 1718238.7, for Oxford University Innovation, dated 3 November 2017; International Patent Application No: PCT/GB2018/053198 for Cambridge Enterprise, based on GB Application No: 1718238.7, dated 5 November 2018. Status: pending. The remaining authors have no conflict of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldstein J.R., Lee R.D. Demographic Perspectives on the Mortality of COVID-19 and Other Epidemics. Proc. Natl. Acad. Sci. USA. 2020;117:22035–22041. doi: 10.1073/pnas.2006392117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z., Wherry E.J. T Cell Responses in Patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiPiazza A.T., Graham B.S., Ruckwardt T.J. T Cell Immunity to SARS-CoV-2 Following Natural Infection and Vaccination. Biochem. Biophys. Res. Commun. 2021;538:211–217. doi: 10.1016/j.bbrc.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schub D., Klemis V., Schneitler S., Mihm J., Lepper P.M., Wilkens H., Bals R., Eichler H., Gartner B.C., Becker S.L., et al. High Levels of SARS-CoV-2-Specific T Cells with Restricted Functionality in Severe Courses of COVID-19. JCI Insight. 2020;5:e142167. doi: 10.1172/jci.insight.142167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng M.H., Zhang S., Porritt R.A., Noval Rivas M., Paschold L., Willscher E., Binder M., Arditi M., Bahar I. Superantigenic Character of an Insert Unique to SARS-CoV-2 Spike Supported by Skewed TCR Repertoire in Patients with Hyperinflammation. Proc. Natl. Acad. Sci. USA. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 7.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redd A.D., Nardin A., Kared H., Bloch E.M., Pekosz A., Laeyendecker O., Abel B., Fehlings M., Quinn T.C., Tobian A.A.R. CD8+ T-Cell Responses in COVID-19 Convalescent Individuals Target Conserved Epitopes from Multiple Prominent SARS-CoV-2 Circulating Variants. Open Forum Infect. Dis. 2021;8:ofab143. doi: 10.1093/ofid/ofab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., Goodwin B., Rubiro P., Sutherland A., Wang E., et al. Impact of SARS-CoV-2 Variants on the Total CD4(+) and CD8(+) T Cell Reactivity in Infected or Vaccinated Individuals. Cell Rep. Med. 2021;2:100355. doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung M.K., Shin E.C. Phenotypes and Functions of SARS-CoV-2-Reactive T Cells. Mol. Cells. 2021;44:401–407. doi: 10.14348/molcells.2021.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsitch M., Grad Y.H., Sette A., Crotty S. Cross-Reactive Memory T Cells and Herd Immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020;20:709–713. doi: 10.1038/s41577-020-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheridan C. COVID-19 Testing Turns to T Cells. Nat. Biotechnol. 2021;39:533–534. doi: 10.1038/s41587-021-00920-9. [DOI] [PubMed] [Google Scholar]
- 14.Shoukat M.S., Foers A.D., Woodmansey S., Evans S.C., Fowler A., Soilleux E.J. Use of Machine Learning to Identify a T Cell Response to SARS-CoV-2. Cell Rep. Med. 2021;2:100192. doi: 10.1016/j.xcrm.2021.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delves P.J., Roitt I.M. The Immune System. First of two parts. N. Engl. J. Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 16.Delves P.J., Roitt I.M. The Immune System. Second of two parts. N. Engl. J. Med. 2000;343:108–117. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 17.Foers A.D., Shoukat M.S., Welsh O.E., Donovan K., Petry R., Evans S.C., FitzPatrick M.E., Collins N., Klenerman P., Fowler A., et al. Classification of Intestinal T-Cell Receptor Repertoires Using Machine Learning Methods Can Identify Patients with Coeliac Disease Regardless of Dietary Gluten Status. J. Pathol. 2021;253:279–291. doi: 10.1002/path.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez L., Beckford J., Alachkar H. Deciphering the TCR Repertoire to Solve the COVID-19 Mystery. Trends Pharmacol. Sci. 2020;41:518–530. doi: 10.1016/j.tips.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamedov I.Z., Britanova O.V., Zvyagin I.V., Turchaninova M.A., Bolotin D.A., Putintseva E.V., Lebedev Y.B., Chudakov D.M. Preparing Unbiased T-Cell Receptor and Antibody CDNA Libraries for the Deep next Generation Sequencing Profiling. Front. Immunol. 2013;4:456. doi: 10.3389/fimmu.2013.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng G., Huang Y., Huang Y., Lyu Z., Lesniak D., Randhawa P. Antigen-Specificity of T Cell Infiltrates in Biopsies with T Cell–Mediated Rejection and BK Polyomavirus Viremia: Analysis by Next Generation Sequencing. Am. J. Transplant. 2016;16:3131–3138. doi: 10.1111/ajt.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howie B., Sherwood A.M., Berkebile A.D., Berka J., Emerson R.O., Williamson D.W., Kirsch I., Vignali M., Rieder M.J., Carlson C.S., et al. High-Throughput Pairing of T Cell Receptor α and β Sequences. Sci. Transl. Med. 2015;7:301ra131. doi: 10.1126/scitranslmed.aac5624. [DOI] [PubMed] [Google Scholar]
- 22.Sims J.S., Grinshpun B., Feng Y., Ung T.H., Neira J.A., Samanamud J.L., Canoll P., Shen Y., Sims P.A., Bruce J.N. Diversity and Divergence of the Glioma-Infiltrating T-Cell Receptor Repertoire. Proc. Natl. Acad. Sci. USA. 2016;113:E3529–E3537. doi: 10.1073/pnas.1601012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossetti M., Spreafico R., Consolaro A., Leong J.Y., Chua C., Massa M., Saidin S., Magni-Manzoni S., Arkachaisri T., Wallace C.A., et al. TCR Repertoire Sequencing Identifies Synovial Treg Cell Clonotypes in the Bloodstream during Active Inflammation in Human Arthritis. Ann. Rheum. Dis. 2017;76:435–441. doi: 10.1136/annrheumdis-2015-208992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziubianau M., Hecht J., Kuchenbecker L., Sattler A., Stervbo U., Rödelsperger C., Nickel P., Neumann A.U., Robinson P.N., Mundlos S., et al. TCR Repertoire Analysis by Next Generation Sequencing Allows Complex Differential Diagnosis of T Cell–Related Pathology. Am. J. Transplant. 2013;13:2842–2854. doi: 10.1111/ajt.12431. [DOI] [PubMed] [Google Scholar]
- 25.Okino S.T., Kong M., Sarras H., Wang Y. Evaluation of Bias Associated with High-Multiplex, Target-Specific Pre-Amplification. Biomol. Detect. Quantif. 2016;6:13–21. doi: 10.1016/j.bdq.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndifon W., Gal H., Shifrut E., Aharoni R., Yissachar N., Waysbort N., Reich-Zeliger S., Arnon R., Friedman N. Chromatin Conformation Governs T-Cell Receptor Jβ Gene Segment Usage. Proc. Natl. Acad. Sci. USA. 2012;109:15865–15870. doi: 10.1073/pnas.1203916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madi A., Shifrut E., Reich-Zeliger S., Gal H., Best K., Ndifon W., Chain B., Cohen I.R., Friedman N. T-Cell Receptor Repertoires Share a Restricted Set of Public and Abundant CDR3 Sequences That Are Associated with Self-Related Immunity. Genome Res. 2014;24:1603–1612. doi: 10.1101/gr.170753.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akatsuka Y., Martin E.G., Madonik A., Barsoukov A.A., Hansen J.A. Rapid Screening of T-Cell Receptor (TCR) Variable Gene Usage by Multiplex PCR: Application for Assessment of Clonal Composition. Tissue Antigens. 1999;53:122–134. doi: 10.1034/j.1399-0039.1999.530202.x. [DOI] [PubMed] [Google Scholar]
- 29.Hettinger K., Fischer S., Panzer S., Panzer-Grümayer E.R. Multiplex PCR for TCR Delta Rearrangements: A Rapid and Specific Approach for the Detection and Identification of Immature and Mature Rearrangements in ALL. Br. J. Haematol. 1998;102:1050–1054. doi: 10.1046/j.1365-2141.1998.00879.x. [DOI] [PubMed] [Google Scholar]
- 30.Shugay M., Britanova O.V., Merzlyak E.M., Turchaninova M.A., Mamedov I.Z., Tuganbaev T.R., Bolotin D.A., Staroverov D.B., Putintseva E.V., Plevova K., et al. Towards Error-Free Profiling of Immune Repertoires. Nat. Methods. 2014;11:653–655. doi: 10.1038/nmeth.2960. [DOI] [PubMed] [Google Scholar]
- 31.Peng Q., Vijaya Satya R., Lewis M., Randad P., Wang Y. Reducing Amplification Artifacts in High Multiplex Amplicon Sequencing by Using Molecular Barcodes. BMC Genom. 2015;16:589. doi: 10.1186/s12864-015-1806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turchaninova M.A., Davydov A., Britanova O.V., Shugay M., Bikos V., Egorov E.S., Kirgizova V.I., Merzlyak E.M., Staroverov D.B., Bolotin D.A., et al. High-Quality Full-Length Immunoglobulin Profiling with Unique Molecular Barcoding. Nat. Protoc. 2016;11:1599–1616. doi: 10.1038/nprot.2016.093. [DOI] [PubMed] [Google Scholar]
- 33.Rapid Amplification of 5′ Complementary DNA Ends (5′RACE) Nat. Methods. 2005;2:629–630. doi: 10.1038/nmeth0805-629. [DOI] [PubMed] [Google Scholar]
- 34.Freeman L.A. Cloning Full-Length Transcripts and Transcript Variants Using 5′ and 3′ RACE. Methods Mol. Biol. 2013;1027:3–17. doi: 10.1007/978-1-60327-369-5_1. [DOI] [PubMed] [Google Scholar]
- 35.Matz M., Shagin D., Bogdanova E., Britanova O., Lukyanov S., Diatchenko L., Chenchik A. Amplification of CDNA Ends Based on Template-Switching Effect and Step-out PCR. Nucleic Acids Res. 1999;27:1558–1560. doi: 10.1093/nar/27.6.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H., He L., Cai L. Transcriptome Sequencing: RNA-Seq. In: Huang T., editor. Computational Systems Biology: Methods and Protocols. Springer; New York, NY, USA: 2018. pp. 15–27. [DOI] [PubMed] [Google Scholar]
- 37.Linnemann C., Heemskerk B., Kvistborg P., Kluin R.J.C., Bolotin D.A., Chen X., Bresser K., Nieuwland M., Schotte R., Michels S., et al. High-Throughput Identification of Antigen-Specific TCRs by TCR Gene Capture. Nat. Med. 2013;19:1534–1541. doi: 10.1038/nm.3359. [DOI] [PubMed] [Google Scholar]
- 38.Mulder D.T., Mahé E.R., Dowar M., Hanna Y., Li T., Nguyen L.T., Butler M.O., Hirano N., Delabie J., Ohashi P.S., et al. CapTCR-Seq: Hybrid Capture for T-Cell Receptor Repertoire Profiling. Blood Adv. 2018;2:3506–3514. doi: 10.1182/bloodadvances.2017014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozarewa I., Armisen J., Gardner A.F., Slatko B.E., Hendrickson C.L. Overview of Target Enrichment Strategies. Curr. Protoc. Mol. Biol. 2015;112:7.21.1–7.21.23. doi: 10.1002/0471142727.mb0721s112. [DOI] [PubMed] [Google Scholar]
- 40.Mamanova L., Coffey A.J., Scott C.E., Kozarewa I., Turner E.H., Kumar A., Howard E., Shendure J., Turner D.J. Target-Enrichment Strategies for next-Generation Sequencing. Nat. Methods. 2010;7:111–118. doi: 10.1038/nmeth.1419. [DOI] [PubMed] [Google Scholar]
- 41.Linnemann C., Mezzadra R., Schumacher T.N.M. TCR Repertoires of Intratumoral T-Cell Subsets. Immunol. Rev. 2014;257:72–82. doi: 10.1111/imr.12140. [DOI] [PubMed] [Google Scholar]
- 42.Looney T.J., Topacio-Hall D., Lowman G., Conroy J., Morrison C., Oh D., Fong L., Zhang L. TCR Convergence in Individuals Treated With Immune Checkpoint Inhibition for Cancer. Front. Immunol. 2019;10:2985. doi: 10.3389/fimmu.2019.02985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang H., Yamaguchi R., Liu X., Daigo Y., Yew P.Y., Tanikawa C., Matsuda K., Imoto S., Miyano S., Nakamura Y. Quantitative T Cell Repertoire Analysis by Deep CDNA Sequencing of T Cell Receptor α and β Chains Using Next-Generation Sequencing (NGS) OncoImmunology. 2014;3:e968467. doi: 10.4161/21624011.2014.968467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lay L., Stroup B., Payton J.E. Validation and Interpretation of IGH and TCR Clonality Testing by Ion Torrent S5 NGS for Diagnosis and Disease Monitoring in B and T Cell Cancers. Pract. Lab. Med. 2020;22:e00191. doi: 10.1016/j.plabm.2020.e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Schaik B., Klarenbeek P., Doorenspleet M., van Kampen A., Moody D.B., de Vries N., Van Rhijn I. Discovery of Invariant T Cells by Next-Generation Sequencing of the Human TCR α-Chain Repertoire. J. Immunol. 2014;193:5338–5344. doi: 10.4049/jimmunol.1401380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitaura K., Shini T., Matsutani T., Suzuki R. A New High-Throughput Sequencing Method for Determining Diversity and Similarity of T Cell Receptor (TCR) α and β Repertoires and Identifying Potential New Invariant TCR α Chains. BMC Immunol. 2016;17:38. doi: 10.1186/s12865-016-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosati E., Dowds C.M., Liaskou E., Henriksen E.K.K., Karlsen T.H., Franke A. Overview of Methodologies for T-Cell Receptor Repertoire Analysis. BMC Biotechnol. 2017;17:61. doi: 10.1186/s12896-017-0379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barennes P., Quiniou V., Shugay M., Egorov E.S., Davydov A.N., Chudakov D.M., Uddin I., Ismail M., Oakes T., Chain B., et al. Benchmarking of T Cell Receptor Repertoire Profiling Methods Reveals Large Systematic Biases. Nat. Biotechnol. 2021;39:236–245. doi: 10.1038/s41587-020-0656-3. [DOI] [PubMed] [Google Scholar]
- 49.Glanville J., Huang H., Nau A., Hatton O., Wagar L.E., Rubelt F., Ji X., Han A., Krams S.M., Pettus C., et al. Identifying Specificity Groups in the T Cell Receptor Repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greiff V., Miho E., Menzel U., Reddy S.T. Bioinformatic and Statistical Analysis of Adaptive Immune Repertoires. Trends Immunol. 2015;36:738–749. doi: 10.1016/j.it.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Bolotin D.A., Poslavsky S., Mitrophanov I., Shugay M., Mamedov I.Z., Putintseva E.V., Chudakov D.M. MiXCR: Software for Comprehensive Adaptive Immunity Profiling. Nat. Methods. 2015;12:380–381. doi: 10.1038/nmeth.3364. [DOI] [PubMed] [Google Scholar]
- 52.Ye J., Ma N., Madden T.L., Ostell J.M. IgBLAST: An Immunoglobulin Variable Domain Sequence Analysis Tool. Nucleic Acids Res. 2013;41:W34–W40. doi: 10.1093/nar/gkt382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alamyar E., Duroux P., Lefranc M.-P., Giudicelli V. IMGT® Tools for the Nucleotide Analysis of Immunoglobulin (IG) and T Cell Receptor (TR) V-(D)-J Repertoires, Polymorphisms, and IG Mutations: IMGT/V-QUEST and IMGT/HighV-QUEST for NGS. In: Christiansen F.T., Tait B.D., editors. Immunogenetics: Methods and Applications in Clinical Practice. Humana Press; Totowa, NJ, USA: 2012. pp. 569–604. Methods in Molecular, Biology. [Google Scholar]
- 54.Smakaj E., Babrak L., Ohlin M., Shugay M., Briney B., Tosoni D., Galli C., Grobelsek V., D’Angelo I., Olson B., et al. Benchmarking Immunoinformatic Tools for the Analysis of Antibody Repertoire Sequences. Bioinformatics. 2020;36:1731–1739. doi: 10.1093/bioinformatics/btz845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shugay M., Bagaev D.V., Turchaninova M.A., Bolotin D.A., Britanova O.V., Putintseva E.V., Pogorelyy M.V., Nazarov V.I., Zvyagin I.V., Kirgizova V.I., et al. VDJtools: Unifying Post-Analysis of T Cell Receptor Repertoires. PLoS Comput. Biol. 2015;11:e1004503. doi: 10.1371/journal.pcbi.1004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ni Q., Zhang J., Zheng Z., Chen G., Christian L., Grönholm J., Yu H., Zhou D., Zhuang Y., Li Q.-J., et al. VisTCR: An Interactive Software for T Cell Repertoire Sequencing Data Analysis. Front. Genet. 2020;11:771. doi: 10.3389/fgene.2020.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bystry V., Reigl T., Krejci A., Demko M., Hanakova B., Grioni A., Knecht H., Schlitt M., Dreger P., Sellner L., et al. ARResT/Interrogate: An Interactive Immunoprofiler for IG/TR NGS Data. Bioinformatics. 2017;33:435–437. doi: 10.1093/bioinformatics/btw634. [DOI] [PubMed] [Google Scholar]
- 58.Scott J.K., Breden F. The Adaptive Immune Receptor Repertoire Community as a Model for FAIR Stewardship of Big Immunology Data. Curr. Opin. Syst. Biol. 2020;24:71–77. doi: 10.1016/j.coisb.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pannetier C., Even J., Kourilsky P. T-Cell Repertoire Diversity and Clonal Expansions in Normal and Clinical Samples. Immunol. Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 60.Li B., Li T., Pignon J.-C., Wang B., Wang J., Shukla S.A., Dou R., Chen Q., Hodi F.S., Choueiri T.K., et al. Landscape of Tumor-Infiltrating T Cell Repertoire of Human Cancers. Nat. Genet. 2016;48:725–732. doi: 10.1038/ng.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui J.-H., Lin K.-R., Yuan S.-H., Jin Y.-B., Chen X.-P., Su X.-K., Jiang J., Pan Y.-M., Mao S.-L., Mao X.-F., et al. TCR Repertoire as a Novel Indicator for Immune Monitoring and Prognosis Assessment of Patients With Cervical Cancer. Front. Immunol. 2018;9:2729. doi: 10.3389/fimmu.2018.02729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolotin D.A., Mamedov I.Z., Britanova O.V., Zvyagin I.V., Shagin D., Ustyugova S.V., Turchaninova M.A., Lukyanov S., Lebedev Y.B., Chudakov D.M. Next Generation Sequencing for TCR Repertoire Profiling: Platform-Specific Features and Correction Algorithms. Eur. J. Immunol. 2012;42:3073–3083. doi: 10.1002/eji.201242517. [DOI] [PubMed] [Google Scholar]
- 63.Postow M.A., Manuel M., Wong P., Yuan J., Dong Z., Liu C., Perez S., Tanneau I., Noel M., Courtier A., et al. Peripheral T Cell Receptor Diversity Is Associated with Clinical Outcomes Following Ipilimumab Treatment in Metastatic Melanoma. J. Immunother. Cancer. 2015;3:25. doi: 10.1186/s40425-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang P., Xu Z., Zhou W., Jin X., Xu C., Luo M., Ma K., Cao H., Huang Y., Lin X., et al. Identification of Potential Vaccine Targets for COVID-19 by Combining Single-Cell and Bulk TCR Sequencing. Clin. Transl. Med. 2021;11:e430. doi: 10.1002/ctm2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keylock C.J. Simpson Diversity and the Shannon–Wiener Index as Special Cases of a Generalized Entropy. Oikos. 2005;109:203–207. doi: 10.1111/j.0030-1299.2005.13735.x. [DOI] [Google Scholar]
- 66.Simpson E.H. Measurement of Diversity. Nature. 1949;163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 67.Venturi V., Kedzierska K., Turner S.J., Doherty P.C., Davenport M.P. Methods for Comparing the Diversity of Samples of the T Cell Receptor Repertoire. J. Immunol. Methods. 2007;321:182–195. doi: 10.1016/j.jim.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Magurran A.E. Measuring Biological Diversity. John Wiley & Sons; Hoboken, NJ, USA: 2013. [Google Scholar]
- 69.Shannon C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948;27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 70.Stewart J.J., Lee C.Y., Ibrahim S., Watts P., Shlomchik M., Weigert M., Litwin S. A Shannon Entropy Analysis of Immunoglobulin and T Cell Receptor. Mol. Immunol. 1997;34:1067–1082. doi: 10.1016/S0161-5890(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 71.Keane C., Gould C., Jones K., Hamm D., Talaulikar D., Ellis J., Vari F., Birch S., Han E., Wood P., et al. The T-Cell Receptor Repertoire Influences the Tumor Microenvironment and Is Associated with Survival in Aggressive B-Cell Lymphoma. Clin. Cancer Res. 2017;23:1820–1828. doi: 10.1158/1078-0432.CCR-16-1576. [DOI] [PubMed] [Google Scholar]
- 72.Willis A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019;10:2407. doi: 10.3389/fmicb.2019.02407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology. 1973;54:427–432. doi: 10.2307/1934352. [DOI] [Google Scholar]
- 74.Greiff V., Bhat P., Cook S.C., Menzel U., Kang W., Reddy S.T. A Bioinformatic Framework for Immune Repertoire Diversity Profiling Enables Detection of Immunological Status. Genome Med. 2015;7:49. doi: 10.1186/s13073-015-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaplinsky J., Arnaout R. Robust Estimates of Overall Immune-Repertoire Diversity from High-Throughput Measurements on Samples. Nat. Commun. 2016;7:11881. doi: 10.1038/ncomms11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mora T., Walczak A.M., Bialek W., Callan C.G. Maximum Entropy Models for Antibody Diversity. Proc. Natl. Acad. Sci. USA. 2010;107:5405–5410. doi: 10.1073/pnas.1001705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gielis S., Moris P., Bittremieux W., De Neuter N., Ogunjimi B., Laukens K., Meysman P. Identification of Epitope-Specific T Cells in T-Cell Receptor Repertoires. Methods Mol. Biol. 2020;2120:183–195. doi: 10.1007/978-1-0716-0327-7_13. [DOI] [PubMed] [Google Scholar]
- 78.Montemurro A., Schuster V., Povlsen H.R., Bentzen A.K., Jurtz V., Chronister W.D., Crinklaw A., Hadrup S.R., Winther O., Peters B., et al. NetTCR-2.0 Enables Accurate Prediction of TCR-Peptide Binding by Using Paired TCRα and β Sequence Data. Commun. Biol. 2021;4:1060. doi: 10.1038/s42003-021-02610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tickotsky N., Sagiv T., Prilusky J., Shifrut E., Friedman N. McPAS-TCR: A Manually Curated Catalogue of Pathology-Associated T Cell Receptor Sequences. Bioinformatics. 2017;33:2924–2929. doi: 10.1093/bioinformatics/btx286. [DOI] [PubMed] [Google Scholar]
- 80.Klinger M., Pepin F., Wilkins J., Asbury T., Wittkop T., Zheng J., Moorhead M., Faham M. Multiplex Identification of Antigen-Specific T Cell Receptors Using a Combination of Immune Assays and Immune Receptor Sequencing. PLoS ONE. 2015;10:e0141561. doi: 10.1371/journal.pone.0141561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shugay M., Bagaev D.V., Zvyagin I.V., Vroomans R.M., Crawford J.C., Dolton G., Komech E.A., Sycheva A.L., Koneva A.E., Egorov E.S., et al. VDJdb: A Curated Database of T-Cell Receptor Sequences with Known Antigen Specificity. Nucleic Acids Res. 2018;46:D419–D427. doi: 10.1093/nar/gkx760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H., Zhan X., Li B. GIANA Allows Computationally-Efficient TCR Clustering and Multi-Disease Repertoire Classification by Isometric Transformation. Nat. Commun. 2021;12:4699. doi: 10.1038/s41467-021-25006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pogorelyy M.V., Minervina A.A., Shugay M., Chudakov D.M., Lebedev Y.B., Mora T., Walczak A.M. Detecting T Cell Receptors Involved in Immune Responses from Single Repertoire Snapshots. PLoS Biol. 2019;17:e3000314. doi: 10.1371/journal.pbio.3000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valkiers S., Van Houcke M., Laukens K., Meysman P. ClusTCR: A Python Interface for Rapid Clustering of Large Sets of CDR3 Sequences with Unknown Antigen Specificity. Bioinformatics. 2021;37:4865–4867. doi: 10.1093/bioinformatics/btab446. [DOI] [PubMed] [Google Scholar]
- 85.Huang H., Wang C., Rubelt F., Scriba T.J., Davis M.M. Analyzing the Mycobacterium Tuberculosis Immune Response by T-Cell Receptor Clustering with GLIPH2 and Genome-Wide Antigen Screening. Nat. Biotechnol. 2020;38:1194–1202. doi: 10.1038/s41587-020-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H., Liu L., Zhang J., Chen J., Ye J., Shukla S., Qiao J., Zhan X., Chen H., Wu C.J., et al. Investigation of Antigen-Specific T-Cell Receptor Clusters in Human Cancers. Clin. Cancer Res. 2020;26:1359–1371. doi: 10.1158/1078-0432.CCR-19-3249. [DOI] [PubMed] [Google Scholar]
- 87.Dash P., Fiore-Gartland A.J., Hertz T., Wang G.C., Sharma S., Souquette A., Crawford J.C., Clemens E.B., Nguyen T.H.O., Kedzierska K., et al. Quantifiable Predictive Features Define Epitope-Specific T Cell Receptor Repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sidhom J.-W., Bessell C.A., Havel J.J., Kosmides A., Chan T.A., Schneck J.P. ImmunoMap: A Bioinformatics Tool for T-Cell Repertoire Analysis. Cancer Immunol. Res. 2018;6:151–162. doi: 10.1158/2326-6066.CIR-17-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajeh A., Wolf K., Schiebout C., Sait N., Kosfeld T., DiPaolo R.J., Ahn T.-H. ICAT: Diagnostic Assessment Tool of Immunological History Using High-Throughput T-Cell Receptor Sequencing. F1000Research. 2021;10:65. doi: 10.12688/f1000research.27214.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henikoff S., Henikoff J.G. Amino Acid Substitution Matrices from Protein Blocks. Proc. Natl. Acad. Sci. USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sidhom J.-W., Larman H.B., Pardoll D.M., Baras A.S. DeepTCR Is a Deep Learning Framework for Revealing Sequence Concepts within T-Cell Repertoires. Nat. Commun. 2021;12:1605. doi: 10.1038/s41467-021-21879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sidhom J.-W., Baras A.S. Deep Learning Identifies Antigenic Determinants of Severe SARS-CoV-2 Infection within T-Cell Repertoires. Sci. Rep. 2021;11:14275. doi: 10.1038/s41598-021-93608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yohannes D.A., Kaukinen K., Kurppa K., Saavalainen P., Greco D. Clustering Based Approach for Population Level Identification of Condition-Associated T-Cell Receptor β-Chain CDR3 Sequences. BMC Bioinform. 2021;22:159. doi: 10.1186/s12859-021-04087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shomuradova A.S., Vagida M.S., Sheetikov S.A., Zornikova K.V., Kiryukhin D., Titov A., Peshkova I.O., Khmelevskaya A., Dianov D.V., Malasheva M., et al. SARS-CoV-2 Epitopes Are Recognized by a Public and Diverse Repertoire of Human T Cell Receptors. Immunity. 2020;53:1245–1257.e5. doi: 10.1016/j.immuni.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schultheiß C., Paschold L., Simnica D., Mohme M., Willscher E., von Wenserski L., Scholz R., Wieters I., Dahlke C., Tolosa E., et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity. 2020;53:442–455.e4. doi: 10.1016/j.immuni.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niu X., Li S., Li P., Pan W., Wang Q., Feng Y., Mo X., Yan Q., Ye X., Luo J., et al. Longitudinal Analysis of T and B Cell Receptor Repertoire Transcripts Reveal Dynamic Immune Response in COVID-19 Patients. Front. Immunol. 2020;11:582010. doi: 10.3389/fimmu.2020.582010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang C.-M., Feng P.-H., Wu T.-H., Alachkar H., Lee K.-Y., Chang W.-C. Profiling of T Cell Repertoire in SARS-CoV-2-Infected COVID-19 Patients Between Mild Disease and Pneumonia. J. Clin. Immunol. 2021;41:1131–1145. doi: 10.1007/s10875-021-01045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minervina A.A., Komech E.A., Titov A., Bensouda Koraichi M., Rosati E., Mamedov I.Z., Franke A., Efimov G.A., Chudakov D.M., Mora T., et al. Longitudinal High-Throughput TCR Repertoire Profiling Reveals the Dynamics of T-Cell Memory Formation after Mild COVID-19 Infection. eLife. 2021;10:e63502. doi: 10.7554/eLife.63502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swanson P.A., II, Padilla M., Hoyland W., McGlinchey K., Fields P.A., Bibi S., Faust S.N., McDermott A.B., Lambe T., Pollard A.J., et al. AZD1222/ChAdOx1 NCoV-19 Vaccination Induces a Polyfunctional Spike Protein-Specific Th1 Response with a Diverse TCR Repertoire. Sci. Transl. Med. 2021;13:eabj7211. doi: 10.1126/scitranslmed.abj7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y., Duan F., Zhu Z., Yu M., Jia X., Dai H., Wang P., Qiu X., Lu Y., Huang J. Analysis of TCR Repertoire by High-Throughput Sequencing Indicates the Feature of T Cell Immune Response after SARS-CoV-2 Infection. Cells. 2021;11:68. doi: 10.3390/cells11010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu W., He M., Wang X., Sun Q., Kuang M. Specific CD8+ TCR Repertoire Recognizing Conserved Antigens of SARS-CoV-2 in Unexposed Population: A Prerequisite for Broad-Spectrum CD8+ T Cell Immunity. Vaccines. 2021;9:1093. doi: 10.3390/vaccines9101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simnica D., Schultheiß C., Mohme M., Paschold L., Willscher E., Fitzek A., Püschel K., Matschke J., Ciesek S., Sedding D.G., et al. Landscape of T-Cell Repertoires with Public COVID-19-Associated T-Cell Receptors in Pre-Pandemic Risk Cohorts. Clin. Transl. Immunol. 2021;10:e1340. doi: 10.1002/cti2.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimizu K., Iyoda T., Sanpei A., Nakazato H., Okada M., Ueda S., Kato-Murayama M., Murayama K., Shirouzu M., Harada N., et al. Identification of TCR Repertoires in Functionally Competent Cytotoxic T Cells Cross-Reactive to SARS-CoV-2. Commun. Biol. 2021;4:1365. doi: 10.1038/s42003-021-02885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Hu J., Wang Y., Liu D., Shi Y., Zhang J., Liu Y., Lin D., Lin J., Hu W., et al. T-Cell Repertoire Characteristics of Asymptomatic and Re-Detectable Positive COVID-19 Patients. Front. Immunol. 2021;12:769442. doi: 10.3389/fimmu.2021.769442. [DOI] [PMC free article] [PubMed] [Google Scholar]