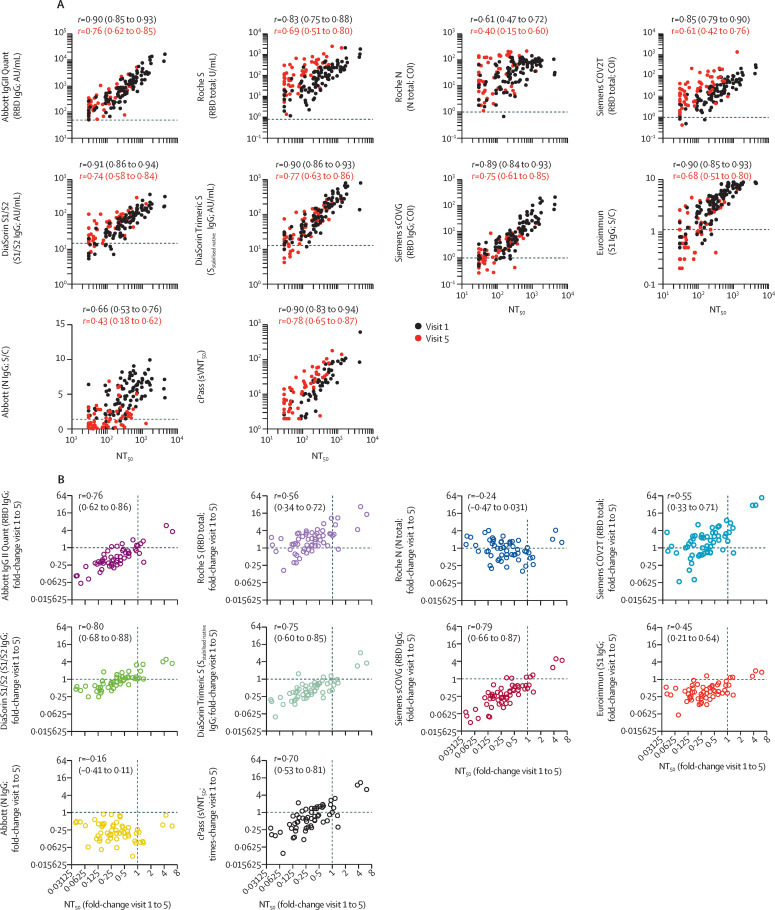
Figure 3.
Correlation of neutralisation titres and serology assays
(A) Correlation of NT50 (x-axis) with serological assay values (y-axis) obtained at visit 1 and visit 5 for each participant; statistical significance was determined with the Spearman correlation test for samples obtained at visit 1 and visit 5 independently, with Spearman's r and respective 95% CIs as indicated; dotted lines indicate serological assay thresholds. (B) Correlation of fold change (visit 1 to visit 5) of NT50s with corresponding fold change in serological assay values for indicated serology assays. Statistical significance was determined using the Spearman correlation test, with Spearman's r and respective 95% CIs as indicated. Dotted lines at x=1 and y=1 indicate unchanged assay results over time. For the cPass assay, the serum dilution at which 50% signal inhibition was achieved was defined as 50% surrogate virus neutralisation titre (sVNT50). AU=arbitrary unit. COI=confidence interval. N=nucleocapsid. NT50=half-maximal neutralisation titre. RBD=receptor binding domain. S=spike protein. S/C=ratio over threshold value. U=unit.