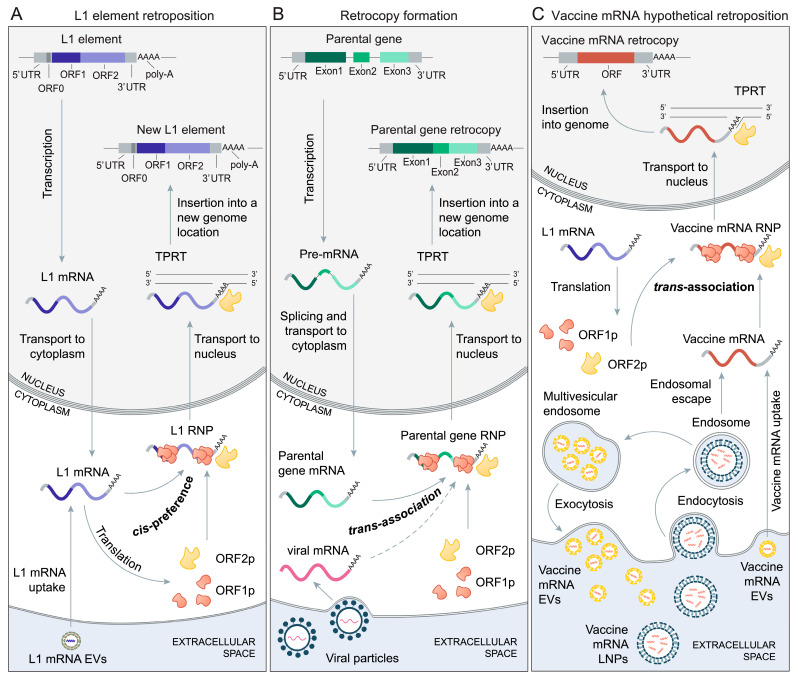
Figure 1.
L1-mediated retroposition. (A) Retroposition cycle of L1 elements. An active L1 element is transcribed in the nucleus and the resulting L1 mRNA is transported to the cytoplasm where it undergoes translation [44,45]. L1 mRNA codes for ORF1 and ORF2 proteins, which preferentially associate with L1 mRNA (cis-preference) to form L1 ribonucleoprotein particle (L1 RNP) [44,45,46]. ORF1p is an RNA binding protein with chaperone activity, while ORF2p functions as reverse transcriptase and endonuclease [47,48]. By a yet unresolved mechanism, L1 RNP, which contains at least L1 mRNA and ORF2p, enters the nucleus. In the nucleus, L1 mRNA is reverse-transcribed and integrated into the genome via the process of target-primed reverse transcription (TPRT) [45,47,48,49]. The retroposition mechanism relies on the binding of ORF2p to the L1 mRNA poly-A tail [48,50,51,52]. There is some evidence that the cells could uptake extracellular vesicles (EVs) containing L1 mRNA, which can then undergo translation and retroposition [53]. (B) L1-mediated retroposition of endogenous coding genes and L1-mediated retroposition of viral mRNAs. A parental protein-coding gene is transcribed in the nucleus. The resulting pre-mRNA is processed and mature parental gene mRNA is then transported to the cytoplasm. L1 proteins (ORF1p and ORF2p) interact with parental gene mRNA via the process termed trans-association to form a parental gene ribonucleoprotein particle (parental gene RNP) [36,45,46,49]. Similar to L1 RNP, a parental gene RNP enters the nucleus where the parental gene mRNA, through TPRT, is reverse-transcribed and integrated into the genome. The poly-A tail of parental gene mRNA plays a crucial role in this process [36,50,51,52]. By a similar process, mRNA molecules that stem from non-retroviral RNA viruses could be integrated into the genome. Examples include the integration of mRNAs from bornaviruses [42,43] and probably coronaviruses [54,55]. (C) Hypothetical L1-mediated retroposition of vaccine mRNA. Vaccine mRNA formulated in lipid nanoparticles (LNPs) enter the cell via endocytosis [1,2,6,10,56]. A fraction of the vaccine mRNA enters the cytosol via endosomal escape, while the rest of the vaccine mRNA undergoes degradation in endosomes [56] or is repackaged in multivesicular endosomes into extracellular vesicles (EVs) and secreted back into the extracellular space [57]. The neighboring or distant cells can uptake vaccine mRNA from these EVs [57,58]. L1 proteins (ORF1p and ORF2p) interact with vaccine mRNA via a process termed trans-association to form a vaccine mRNA ribonucleoprotein particle (vaccine mRNA RNP) [36,45,46,49]. Like L1 and parental gene RNPs, a vaccine mRNA RNP enters the nucleus where the vaccine mRNA, through TPRT, is reverse-transcribed and integrated into the genome. The poly-A tail of vaccine mRNA plays a crucial role in this process [36,50,51,52].