Abstract
In this paper, risk compensation among individuals on antiretroviral therapy (ART), using the 2017 South African national survey on HIV, is explored. A multi-stage stratified cluster random sampling approach was used to realize 11,130 participants 15 years and older. Logistic regression analysis assessed the association between multiple sexual partners, condom use at last sexual encounter, consistency of condom usage and potential explanatory variables using HIV status and ART exposure as a mediator variable. HIV positive participants who were aware and on ART were less likely to have multiple sexual partners, and less likely not to use a condom at last sex compared to HIV positive participants who were aware but not on ART. The odds of reporting multiple sexual partners were significantly lower among older age groups, females, non-Black Africans, and rural settings, and higher among those with tertiary level education, and risky alcohol users. The odds of no condom use at last sexual encounter were more likely among older age groups, females, other race groups, and less likely among those with secondary level education. The odds of inconsistent condom use were more likely among older age groups, females, and other race groups, and less likely among those with tertiary level education, high risk and hazardous alcohol users. Risk compensation is not apparent among HIV infected adults who are on ART. Risk groups that should receive tailored interventions to reduced risky sexual behaviours were identified.
Keywords: HIV, risk compensation, ART, sexual behaviour, South Africa
1. Introduction
South Africa had an estimated HIV prevalence of 14% or approximately 7.9 million people living with HIV in 2017, and, among those aged 15 years and older, HIV presence was higher, at 18.8% [1]. The 2017 survey revealed that among all people living with HIV, 62.3% were receiving antiretroviral therapy (ART) [1]. The proportion of people on treatment increased with age from 15 years up and was highest among people aged 50 years or older at 76.7% [1]. Evidence on treatment as prevention [1,2,3], and the adoption of the universal “test and treat“ as an approach to manage HIV, has placed ART scale-up at the centre of achieving an “AIDS-free generation”, not only in South Africa but also globally [4,5,6,7]. Initiation of ART as early as possible after acquiring HIV has been shown to reduce the risk of HIV transmission to uninfected partners by more than 90% [2,3,4,8] and to significantly improve the survival of people living with HIV (PLHIV) [9]. In addition, effective ART can reduce viremia to undetectable levels and prevent onward HIV transmission.
South Africa has one of the world’s largest ART programmes, with an estimated 70% of PLHIV on ART in 2019 [10]. South Africa adopted a test and treatment model in 2016 [1], and ART scale-up is currently ongoing. The National Department of Health had targeted the initiating of an additional two million people by December 2020 [11]. The enrollment of high numbers of HIV-positive individuals on ART is expected to impact HIV incidence at a population level. However, research suggests that the effects of ART on HIV incidence is dependent on other factors, such as changes in sexual networking dynamics during ART scale-up, adherence to ART, achieving viral suppression and patterns of risky sexual behaviours, including unprotected sex and number of sexual partners [9].
There is an ongoing debate on the effect of increased access to ART on risky sexual behavior. At issue is whether increased access to ART, improved viral suppression, and reduced viral transmission may lead HIV-positive individuals to engage in high-risk sexual behaviours they would have otherwise not engaged in without ART treatment, also known as risk compensation [9]. Risk compensation occurs when people engage in higher individual risk behaviours, such as having multiple sexual partners and/or having unprotected sex, due to the increased availability of interventions to prevent and mitigate HIV and other sexually transmitted infections [12,13,14,15,16]. As we increase the availability of ART, it is important to understand the effects on the sexual behaviours of PLHIV, since this has implications for the spread and control of the HIV epidemic.
Studies on the sexual behaviours of people on ART are inconsistent [17]. However, a significant reduction in risky sexual behaviour among people on ART in sub-Saharan Africa was shown in a meta-analysis [17], though the review could not identify what contributes to the positive behavioural change, and it is unclear whether the observed behavioural changes could be maintained and if it was representative of all sub-Saharan African countries. Other empirical studies have concluded that there was generally limited evidence of risk compensation after ART initiation [9,18,19,20,21,22]. In contrast, others found no difference in sexual risk behaviour, comparing PLHIV on ART and those not on ART [19]. Some studies showed that participants who are not on ART, have more unprotected sexual intercourse than those who are on ART, due to these participants believing that HIV treatment was a sufficient prevention strategy because the risk of transmission is reduced when one is on HIV treatment and viral load has been suppressed [23,24,25]. However, empirical studies have dismissed the increased risk behaviours regarding the perception of reduced HIV transmission risk after ART initiation have largely been dispelled in [9,20,26].
In South Africa, a cohort study found that although unsafe sexual behaviours had decreased among HIV positive individuals after initiation into ART, some proportion did not practice safe sex [18]. It is unclear whether this pattern continues to prevail in South Africa, especially in the context of a massively scaled-up treatment programme and improved survival of patients on ART. This paper explores the question of risk compensation or risky sexual behaviour among individuals on ART in South Africa, using the 2017 South African National HIV Prevalence, Incidence, Behaviour and Communication Survey.
2. Materials and Methods
2.1. Survey Design and Population
The data used in this paper was obtained from a cross-sectional, population-based household HIV survey conducted in 2017, using a multi-stage stratified cluster random sampling approach described in detail elsewhere [1]. In summary, 1000 small area layers (SALs) were sampled using Statistics South Africa’s 2015 national population sampling frame which consisted of 84,907 SALs [27]. The selection of SALs were stratified by province, locality type (urban, rural formal, and rural informal/tribal areas) and race groups in urban areas, based on the predominant race group in the selected SAL. A total of 15 visiting points (VPs)/households were randomly selected from each of the 1000 SALs, targeting 15,000 VPs. Of these, 12,435 (82.9%) VPs were approached due to lack of access in gated, farm and tribal communities. Among these VPs, 11,776 (94.7%) were valid, and a household response rate of 82.2% was achieved from the valid VPs. This survey included people of all ages living in South Africa. All members in the selected households were invited to participate in the survey [1]. The data was benchmarked to the mid-year estimates for 2017 to generalise the findings to the South African population [27].
Informed consent and assent were sought before participants were enrolled in the study. All consenting members of the selected households formed the ultimate sampling unit. A household questionnaire (collected information about the household situation) and three age-appropriate individual questionnaires were used to solicit, among others, sociodemographic information, and sexual history, including HIV related risk behaviours. The questionnaires were administered by field-workers and electronically captured using CSPro software on Mercer tablets.
2.2. Blood Specimen Collection and Processing
The survey also included collecting a blood specimen for estimating HIV prevalence and ART exposure from consenting participants [1]. Dried blood spot (DBS) samples were collected by finger prick from consenting individuals and were tested for HIV antibodies using an algorithm with three different enzyme immunoassays (EIAs). All samples which were HIV positive during the first two EIAs (Roche Elecys HIV Ag/Ab assay, Roche Diagnostics, Mannheim, Germany and Genescreen Ultra HIV Ag/Ab assay, Bio-Rad Laboratories, Hercules, CA, USA) were subjected to a nucleic acid amplification test (COBAS AmpliPrep/Cobas Taqman HIV-1 Qualitative Test, v2.0, Roche Molecular Systems, Branchburg, NJ, USA) for the final interpretation of test results. Testing for exposure to antiretroviral drugs in HIV-positive specimens was performed using High Performance Liquid Chromatography (HPLC), coupled with Tandem Mass Spectrometry [1].
The current study used a sub-sample of data on youth and adults aged 15 years and older who agreed to be tested for HIV, whose blood specimen was screened for the presence of ART, and who responded to the question on awareness of HIV status.
2.3. Measures
2.3.1. Primary Outcome and Control Variables
The primary outcome measure consisted of risky sexual behaviours, including condom use at the last sexual encounter, consistent condom use, and the number of sexual partners in the past 12 months. Condom use at the last sexual encounter was based on the question: “Did you use a condom at last sexual encounter with the most recent person you had sex with?” Consistent condom use was based on the question: “How often do you use a condom with your (1) most recent sexual partner (2) second most recent sexual partner and (3) and third sexual partner? Responses were combined into a composite variable and dichotomised into a binary outcome with 1 = every time, 0 = almost every time, 0 = sometimes and never = 0. Multiple sexual partnership is based on the question: “Overall how many sexual partners did you have during the past 12 months”? Responses were coded and dichotomised into risky sexual behaviour indicators as follows:
Condom use at last sex (No = 1 and Yes = 0)
Consistent condom use (No = 1 and Yes = 0)
Number of sexual partners in the past 12 months (One partner = 0 and Two or more partners = 1).
The mediator variable used HIV positive individuals who were aware of their HIV positive status and not on ART as a reference category:
HIV positive, aware and not ART = 0
HIV negative aware = 1
HIV positive aware, and on ART = 2.
2.3.2. Explanatory Variables
Descriptive measures included socio-demographic characteristics, such as age group in years (15–19, 20–24, 25–49, 50 years and older), race (Black Africans and other race groups, which included Whites, Coloureds, and Indians/Asians), marital status (married and not married), educational level completed (no education, primary, secondary, and tertiary), employment status (not employed and employed), locality type (urban, rural informal, rural formal), and alcohol use measured using the AUDIT risk score (0 = abstainers; 1–7 = low-risk drinkers; 8–19 = high-risk drinkers; 20+ = hazardous drinking) [28], which has been validated in South Africa [29].
2.4. Statistical Analysis
Descriptive analysis summarised the study sample and risky sexual behaviours by socio-demographic and socio-behavioural factors. Chi-square test was used for comparison of categorical variables. Bivariate logistic regression models were used to assess the relationship between condom use at the last sexual encounter, consistent condom use, number of sexual partners in the past 12 months and potential explanatory variables. In addition, statistically significant variables were entered into a multivariate logistic regression analysis to determine factors jointly and independently associated with selected risky sexual behaviour(s). Levels of risky sexual behaviour(s) were compared between HIV positive individuals who were aware of their HIV status, but were not on ART (reference group), and each of the following two groups: (i) HIV negative individuals who were aware of their HIV status, and (ii) HIV positive individuals who were aware of their HIV status and were on ART. Unadjusted and adjusted odds ratio (AOR) with 95% confidence interval (CI) and p-value ≤ 0.05 was used to test for statistical significance. The analysis was weighted to account for the complex multilevel unequal sampling probabilities in the survey design. All analyses were carried out using STATA version 15.0 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Description of the Study Sample
Of 11,130 participants 15 years and older (n = 1343) 13.7% (95% CI: 12.6–15.0) were aware of their HIV positive status and on ART, (n = 645) 7.1% (95% CI: 6.3–7.8) were aware of their HIV positive status and not on ART and (n = 9142) 79.2% (95% CI: 77.8–80.6) were HIV negative and aware of their HIV status. Table 1 shows sample characteristics. The majority of the weighted population were 25–49 years, female, Black African, never married, had secondary level education, unemployed, resided in urban areas, and were abstainers from alcohol. In addition, most participants reported multiple sexual partners in the last 12 months, no condom use at the last sexual encounter, and no consistent condom use.
Table 1.
Participant’s socio-demographic and behavioural characteristics of the study sample, South Africa 2017 survey.
| Variables | N | % |
|---|---|---|
| Age group in years | ||
| 15–24 | 2354 | 19.7 |
| 25–49 | 5764 | 60.4 |
| 50+ | 3023 | 19.9 |
| Sex | ||
| Male | 4046 | 45.1 |
| Female | 7095 | 54.9 |
| Race groups | ||
| Black African | 7856 | 83.1 |
| Other | 3285 | 16.9 |
| Marital status | ||
| Married | 3617 | 33.6 |
| Never married | 6332 | 66.4 |
| Education level | ||
| No education/primary | 1823 | 15.4 |
| Secondary | 5963 | 68.7 |
| Tertiary | 1078 | 15.9 |
| Employment status | ||
| Not employed | 7195 | 62.7 |
| Employed | 3800 | 37.3 |
| Locality type | ||
| Urban | 7211 | 72.2 |
| Rural informal (tribal areas) | 2843 | 24.2 |
| Rural (farms) | 1087 | 3.6 |
| AUDIT score * | ||
| Abstainers | 6944 | 65.9 |
| Low-risk drinkers (1–7) | 2100 | 22.2 |
| High-risk drinkers (8–19) | 928 | 10.3 |
| Hazardous drinkers (20+) | 148 | 1.6 |
| Numbers of sexual partners in the 12 months | ||
| One partner | 6047 | 88.6 |
| Two or more partners | 614 | 11.4 |
| Condom use at last sex | ||
| No | 2360 | 39.0 |
| Yes | 4262 | 61.0 |
| Consistent condom use | ||
| No | 69 | 1.0 |
| Yes | 11,072 | 99.0 |
Subtotals do not all equal to the total (N), due to non-response and/or missing data, * alcohol risk score based on a questionnaire for Alcohol Use Disorder Identification Test (AUDIT).
Table 2 shows the distribution of risky sexual behaviours by socio-demographic and alcohol use characteristics. Reporting of multiple sexual partners in the past year was significantly higher among those 25–49 years old, males, Black African, never married, having secondary level education, residing in urban areas, and being hazardous alcohol drinkers. Reporting of no condom use at last sexual encounter was significantly higher among those 50 years and older, females, other race groups, married couples, participants with tertiary level education, employed, and participants residing in urban areas. Participants who reported consistently not using a condom were significantly higher among those 25–49 years old, females, never married, or being abstinent.
Table 2.
Distribution of risky sexual behaviours by socio-demographic and alcohol use characteristics, South Africa 2017 survey.
| Variables | Multiple Sexual Partners | No Condom Use at Last Sex | No Consistent Condom Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | p-Value | n | % | 95% CI | p-Value | n | % | 95% CI | p-Value | |
| Age group years | ||||||||||||
| 15–24 | 1369 | 18.0 | 15.2–21.2 | <0.001 | 1357 | 43.7 | 40.1–47.4 | <0.001 | 1352 | 97.3 | 95.6–98.3 | 0.014 |
| 25–49 | 4174 | 11.1 | 9.7–12.8 | 4162 | 61.7 | 59.5–63.9 | 4117 | 98.5 | 97.7–99.0 | |||
| 50+ | 1117 | 3.4 | 2.2–5.2 | 1100 | 83.1 | 79.4–86.2 | 1090 | |||||
| Sex | ||||||||||||
| Male | 2636 | 17.7 | 15.6–20.0 | <0.001 | 2630 | 58.0 | 55.4–60.6 | <0.001 | 2601 | 97.1 | 95.9–97.9 | <0.001 |
| Female | 4024 | 5.5 | 4.4–6.8 | 3989 | 64.0 | 61.7–66.2 | 3958 | 99.8 | 99.6–99.9 | |||
| Race groups | ||||||||||||
| African | 4768 | 12.6 | 11.1–14.2 | <0.001 | 4759 | 56.8 | 54.8–58.7 | <0.001 | 4714 | 98.3 | 97.6–98.8 | 0.169 |
| Other | 1892 | 5.5 | 4.1–7.3 | 1860 | 83.8 | 80.9–86.3 | 1845 | 99.1 | 98.0–99.6 | |||
| Marital status | ||||||||||||
| Married | 2622 | 4.1 | 3.0–5.7 | <0.001 | 2602 | 82.1 | 79.3–84.6 | <0.001 | 2574 | 99.9 | 99.6–100.0 | <0.001 |
| Never married | 3767 | 16.1 | 14.4–18.0 | 3746 | 48.9 | 46.5–51.3 | 3718 | 97.5 | 96.6–98.3 | |||
| Education level | ||||||||||||
| No education/primary | 796 | 5.3 | 3.6–7.8 | 0.005 | 798 | 63.3 | 58.2–68.1 | <0.001 | 788 | 99.8 | 99.3–99.9 | 0.149 |
| Secondary | 4049 | 11.6 | 10.2–13.3 | 4024 | 61.0 | 58.7–63.3 | 3992 | 98.6 | 97.7–99.2 | |||
| Tertiary | 769 | 10.1 | 7.3–13.9 | 755 | 73.0 | 68.2–77.4 | 750 | 98.7 | 96.7-99.5 | |||
| Employment status | ||||||||||||
| Not employed | 3910 | 11.9 | 10.4–13.5 | 0.434 | 3890 | 56.7 | 54.4–59.0 | <0.001 | 3859 | 98.6 | 98.0–99.0 | 0.235 |
| Employed | 2681 | 10.9 | 9.1–13.1 | 2663 | 66.6 | 63.8–69.4 | 2634 | 98.3 | 96.9–99.0 | |||
| Locality type | ||||||||||||
| Urban | 4417 | 12.3 | 10.8–14.0 | <0.001 | 4371 | 62.6 | 60.3–64.8 | <0.001 | 4326 | 98.3 | 97.5–98.8 | 0.124 |
| Rural informal (tribal areas) | 1575 | 9.4 | 7.6–11.6 | 1581 | 54.5 | 51.0–58.0 | 1567 | 98.8 | 98.0–99.3 | |||
| Rural (farms areas) | 668 | 5.8 | 3.7–9.0 | 667 | 67.6 | 60.5–74.0 | 666 | 99.6 | 99.0–99.9 | |||
| AUDIT score * | ||||||||||||
| Abstainers | 3751 | 7.8 | 6.5–9.2 | <0.001 | 3728 | 59.8 | 57.4–62.1 | 0.251 | 3697 | 98.9 | 98.2–99.3 | 0.001 |
| Low-risk drinkers (1–7) | 1490 | 12.5 | 9.9–15.8 | 1473 | 63.9 | 60.2–67.5 | 1459 | 98.7 | 96.1–99.6 | |||
| High-risk drinkers (8–19) | 707 | 22.0 | 18.2–26.4 | 709 | 60.1 | 54.9–65.0 | 697 | 97.5 | 95.4-–98.6 | |||
| Hazardous drinkers (20+) | 112 | 39.9 | 26.9–54.5 | 113 | 55.0 | 40.1–69.0 | 112 | 90.4 | 80.4–95.6 | |||
Subtotals do not all equal to the total (n) due to non-response and/or missing data, CI—confidence intervals, * alcohol risk score based on a questionnaire for Alcohol Use Disorder Identification Test (AUDIT).
3.2. Risky Sexual Behaviour by HIV Status and ART Exposure
A comparison of HIV risky sexual behaviours, HIV status and ART exposure is shown in Table 3. Overall, HIV positive participants who were aware of their status and on ART were significantly less likely to have multiple sexual partners in the past 12 months than HIV positive individuals who were aware of their status and not on ART [OR = 0.6 (95% CI: 0.4–0.8), p = 0.001]. HIV-negative individuals who were aware of their status were significantly more likely not to use a condom during their last sexual encounter than HIV positive participants who were aware of their status and not on ART [OR = 1.4 (95% CI: 1.2–1.7), p < 0.001]. HIV positive participants who were aware of their status and on ART were significantly less likely not to use a condom at the time of their last sexual encounter than HIV positive participants who were aware of their status and not on ART [OR = 0.6 (95% CI: 0.5–0.7), p < 0.001]. There was no statistically significant association between HIV status, ART exposure and consistent condom use.
Table 3.
Risky sexual behaviour variables by HIV status and ART exposure among individuals 15 years older, South Africa 2017 survey.
| Variables | Multiple Sexual Partners | No Condom Use at Last Sex | No Consistent Condom Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Values | OR | 95% CI | p-Values | OR | 95% CI | p-Value | ||||
| HIV positive aware and not on ART | 1 | 1 | 1 | |||||||||
| HIV negative and aware | 1.0 | 0.8 | 1.3 | 0.890 | 1.4 | 1.2 | 1.7 | <0.001 | 1.1 | 0.5 | 2.2 | 0.837 |
| HIV positive aware and on ART | 0.6 | 0.4 | 0.8 | 0.001 | 0.6 | 0.5 | 0.7 | <0.001 | 1.8 | 0.7 | 4.4 | 0.224 |
ART—antiretroviral treatment; CI—confidence interval; OR—odd ratio.
3.3. Factors Associated with Risky Sexual Behaviour
3.3.1. Bivariate Logistic Regression Models
Table 4 presents unadjusted bivariate logistic regression models for socio-demographic factors and each of three risky sexual behaviour outcomes using HIV status and ART exposure as a design variable. All statistically significant variables were entered into multivariate logistic regression models. The odds of reporting multiple sexual partners in the past 12 months were significantly lower among older age groups than younger age groups, females than males, other race groups than Black Africans, and those residing in rural informal and formal areas than urban areas. The odds of reporting multiple sexual partners in the past 12 months were significantly higher among participants who were never married than married, those who had higher levels of education than no education or those with primary level education, and those who were alcohol users (low risk, high and hazardous drinkers) than abstainers.
Table 4.
Unadjusted odds ratios for risky sexual behaviours by socio-demographic factors among individuals 15 years older, South Africa 2017 survey.
| Multiple Sexual Partners | No Condom Use at Last Sex | No Consistent Condom Use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Values | OR | 95% CI | p-Values | OR | 95% CI | p-Values | ||||
| Age groups | ||||||||||||
| 15–24 | 1 | 1 | 1 | |||||||||
| 25–49 | 0.6 | 0.5 | 0.7 | <0.001 | 2.4 | 2.2 | 2.6 | <0.001 | 2.2 | 1.5 | 3.3 | <0.001 |
| 50+ | 0.2 | 0.1 | 0.3 | <0.001 | 6.7 | 5.7 | 7.8 | <0.001 | 15.8 | 3.8 | 65.1 | <0.001 |
| Sex | ||||||||||||
| Male | 1 | 1 | 1 | |||||||||
| Female | 0.2 | 0.2 | 0.3 | <0.001 | 1.3 | 1.2 | 1.5 | <0.001 | 10.8 | 6.2 | 18.7 | <0.001 |
| Race | ||||||||||||
| Black African | 1 | 1 | 1 | |||||||||
| Other | 0.5 | 0.4 | 0.6 | <0.001 | 3.0 | 2.6 | 3.4 | <0.001 | 2.1 | 1.1 | 4.0 | 0.018 |
| Marital status | ||||||||||||
| Married | 1 | 1 | 1 | |||||||||
| Never married | 4.3 | 3.6 | 5.3 | <0.001 | 0.2 | 0.2 | 0.2 | <0.001 | 0.1 | 0.0 | 0.2 | <0.001 |
| Educational qualification | ||||||||||||
| None/primary | 1 | 1 | 1 | |||||||||
| Secondary | 1.8 | 1.4 | 2.3 | <0.001 | 0.6 | 0.6 | 0.7 | <0.001 | 0.2 | 0.1 | 0.8 | 0.017 |
| Tertiary | 1.8 | 1.3 | 2.5 | <0.001 | 0.8 | 0.7 | 1.0 | 0.013 | 0.2 | 0.1 | 0.7 | 0.016 |
| Employment status | ||||||||||||
| No | 1 | 1 | 1 | |||||||||
| Yes | 0.9 | 0.8 | 1.1 | 0.222 | 1.43 | 1.22 | 1.43 | <0.001 | 1.02 | 0.69 | 1.50 | 0.938 |
| Locality type | ||||||||||||
| Urban | 1 | 1 | 1 | |||||||||
| Rural informal (tribal areas) | 0.8 | 0.7 | 0.9 | <0.001 | 0.8 | 0.7 | 0.8 | <0.001 | 1.8 | 1.1 | 2.8 | 0.011 |
| Rural (farms) | 0.6 | 0.4 | 0.7 | <0.001 | 1.1 | 1.0 | 1.3 | 0.133 | 2.6 | 1.0 | 6.3 | 0.042 |
| AUDIT score * | ||||||||||||
| Abstainers | 1 | 1 | 1 | |||||||||
| Low risk drinkers (1–7) | 1.9 | 1.6 | 2.3 | <0.001 | 1.0 | 0.9 | 1.2 | 0.438 | 1.0 | 0.5 | 1.8 | 0.900 |
| High risk drinkers (8–19) | 4.6 | 3.9 | 5.5 | <0.001 | 1.0 | 0.8 | 1.1 | 0.516 | 0.2 | 0.1 | 0.3 | <0.001 |
| Hazardous drinkers (20+) | 7.0 | 4.9 | 10.0 | <0.001 | 1.2 | 0.9 | 1.7 | 0.279 | 0.1 | 0.0 | 0.2 | <0.001 |
Subtotals do not all equal to the total (n) due to non-response and/or missing data, OR—odds ratio, CI—confidence intervals, * alcohol risk score based on a questionnaire for Alcohol Use Disorder Identification Test (AUDIT).
The odds of reporting no condom use during the last sexual encounter were significantly lower among those who were never married, those who had higher levels of education, and those residing in rural informal areas. In addition, the odds of reporting no condom at the time of their last sexual encounter were significantly higher among older age groups, females, other race groups, and the employed.
The odds of reporting inconsistent condom use were significantly lower among participants who were never married, those who had higher levels of education, and were alcohol users (low risk, high and hazardous drinkers). In addition, the odds of reporting inconsistent condom use were significantly higher among older age groups, females, other race groups, and those residing in rural informal areas.
3.3.2. Multivariate Logistic Regression Models
Multiple Sexual Partnerships
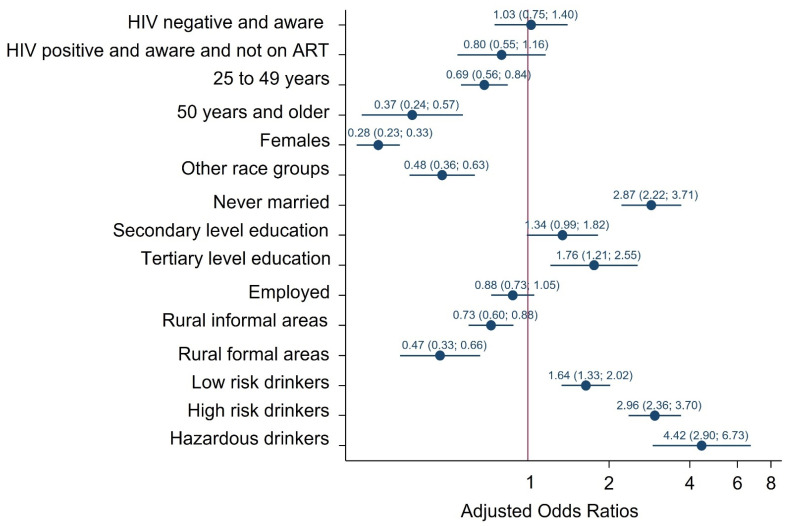
Figure 1 presents multivariate logistic regression models of factors associated with reporting multiple sexual partners in the past 12 months. There was no statistically significant association between HIV status, ART exposure and multiple sexual partners in the past 12 months. The odds of reporting multiple sexual partners in the past 12 months were significantly lower among those aged 25–49 years [AOR = 0.69 (95%CI: 0.56–0.84), p < 0.001], and those 50 years and older [AOR = 0.37 (95% CI: 0.24–0.57), p < 0.001] than youth aged 15–24 years, females than males [AOR = 0.28 (95% CI: 0.23–0.33), p < 0.001], other race groups than Black Africans [AOR = 0.48 (95% CI: 0.36–0.63), p < 0.001], rural informal areas [AOR = 0.73 (95% CI: 0.60–0.88), p = 0.001] and rural formal areas [AOR = 0.47 (95% CI: 0.33–0.66), p < 0.001] than urban areas. The odds of reporting multiple sexual partners in the past 12 months were significantly higher among participants who never married than participants who were married [AOR = 2.87 (95% CI: 2.23–3.71), p < 0.001], participants with tertiary level education rather than no education/primary level education [AOR = 1.76 (95% CI: 1.21–2.56), p = 0.003], low-risk alcohol drinkers [AOR = 1.64 (95% CI: 1.33–2.02), p < 0.001], high-risk alcohol drinkers [AOR = 2.96 (95% CI: 2.37–3.70), p < 0.001] and hazardous alcohol drinkers [AOR = 4.42 (95% CI: 2.91–6.73), p < 0.001] than abstainers.
Figure 1.
Multivariate logistic model of factors associated with reporting multiple sexual partners in the past year among individuals 15 years older, South Africa 2017 survey.
Condom Use at the Last Sexual Encounter
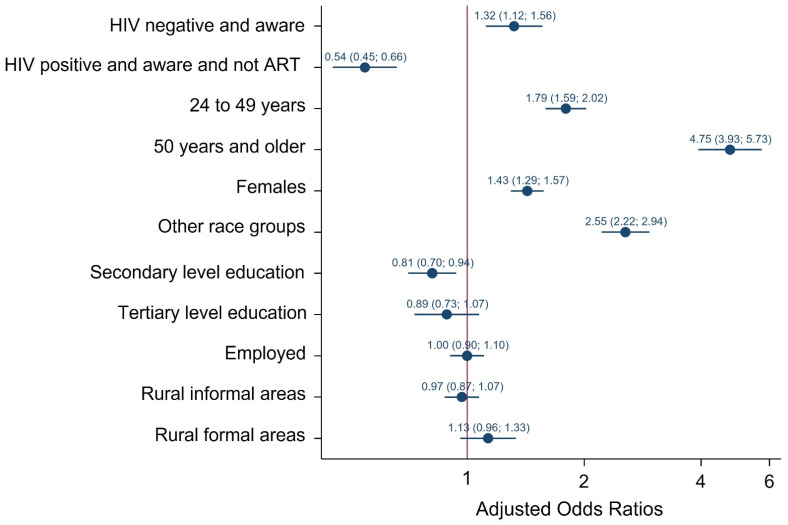
Figure 2 presents multivariate logistic regression models of factors associated with not using a condom at the last sexual encounter. Relative to HIV positive participants not on ART the odds of not using a condom at the time of their last sexual encounter were significantly higher among HIV negative participants who were aware [AOR = 1.32, CI: 1.12–1.56), p = 0.001] and significantly lower among HIV positive participants who were on ART [AOR = 0.54 (95 CI: 0.45–0.66), p < 0.001]. The odds of reporting no condom use at the time of their last sexual encounter were significantly lower among participants with secondary level education than participants with no education/primary level education [AOR = 0.81 (95% CI: 0.70–0.94), p = 0.004]. The odds of reporting no condom use at the time of their last sexual encounter were significantly higher among those aged 25–49 years [AOR = 1.79 (95% CI:1.59–2.02), p < 0.001], 50 years and older [AOR = 4.75 (95% CI: 3.93–7.73), p < 0.001] than youth 15–24 years, females than males [AOR1.43 = (95% CI: 1.29–1.57), p < 0.001], other race groups than Black African [AOR = 2.55 (95% CI: 2.22–2.94), p < 0.001].
Figure 2.
Multivariate logistic model of factors associated with not using a condom during their last sexual encounter among individuals 15 years older, South Africa 2017 survey.
Consistent Condom Use
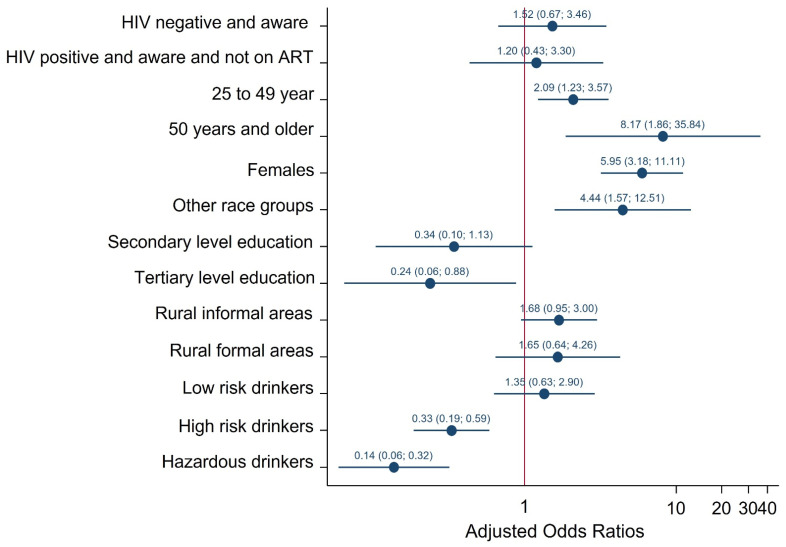
Figure 3 shows multivariate logistic regression models of factors associated with no consistent condom use. There was no statistically significant association between HIV status, ART exposure and no consistent condom use. The odds of reporting no consistent condom were significantly lower among participants with tertiary level education than no education/primary level education [AOR = 0.23 (95% CI: 0.06–0.88), p = 0.031], high-risk alcohol drinker [AOR = 0.33 (95% CI: 0.19–0.59), p < 0.001] and hazardous alcohol drinkers [AOR = 0.14 (95% CI: 0.06–0.32), p < 0.001] than participants who abstained. The odds of reporting no condom use at the time of their last sexual encounter were significantly higher among participants aged 25–49 years [AOR = 2.09 (95% CI: 1.23–3.57), p = 0.007], 50 years and older [AOR = 8.17 (95% CI: 1.86–35.84), p = 0.005] than youth 15–24 years, females than males [AOR = 5.95 (95% CI: 3.18–11.11), p < 0.001], other race groups than Black African [AOR = 4.44 (95% CI: 1.57–12.51), p = 0.005] and rural informal areas than urban areas [AOR = 1.68 (95% CI: 0.95–3.00), p = 0.076].
Figure 3.
Multivariate logistic model of factors associated with no consistent condom use during their last sexual encounter among individuals 15 years older, South Africa 2017 survey.
4. Discussion
The results of this nationally representative population-based study revealed significantly reduced risk behaviours among individuals that were aware of their HIV positive status and also on ART compared to participants who were HIV positive but not on ART. The results do not provide evidence of risk compensation due to exposure to ART as other studies have suggested [19]. Other studies have also reported reductions in risky sexual behaviour after ART initiation [8,17]. Evidence suggests that HIV counselling and support, associated with engagement with healthcare by people on treatment, help these individuals to limit their risk-taking [30]. Literature suggests a complex relationship between the introduction of ART and change in perceptions of HIV risk, and subsequent changes in behaviour. Others suggest that the heterogeneity of published literature reflects different study designs (longitudinal studies, cohorts, cross-sectional surveys), different study populations (heterosexual couples, key populations, drug users) and different socio-cultural contexts [31]. Therefore the current study contributes to the growing body of literature on the sexual behaviour of people on ART in sub-Saharan Africa; especially important, given mixed and contradictory findings on this topic in the continent.
In this study a few variables were used as indicators of risky behaviour, including multiple sexual partners, condom use at the last sexual encounter and consistent condom use. Reporting multiple sexual partners was significantly less likely among older age groups, females, other race groups, and rural settings, and more likely among those who never married, those with tertiary level education, and alcohol users. Multiple sexual partnerships are one of the sexual risk behaviours placing young people, and especially unmarried men, at risk of HIV infection [32,33]. In addition, educational attainment and alcohol consumption have been identified as being associated with multiple sexual partnerships [34,35].
Consistent with current findings, other studies also found substantial variations in condom use behaviour and sociodemographic characteristics. For example, patterns of condom use were significantly less likely among older age groups, females, and other race groups [36,37,38,39,40,41,42]. Power dynamics and type of partner play significant roles in age and gender differences in the pattern of condom use [43,44,45,46]. Studies have shown that condom use, and consistent condom use, were less likely among women, especially among females with an older partner [47]. In such relationships, the older partners are more likely to be the decision makers than their younger or same age partners and they are also less likely to use condoms [47]. Similarly, studies from sub-Saharan Africa have shown the relationship between gender and condom use, where men are more likely to report consistent condom use [48,49,50]. The exact nature of these differences has yet to be elucidated through research studies [49]. Others deduce that gender and relationship constructs are associated with condom use patterns, particularly the masculine nature of male gender identity [51].
The findings also indicate that lack of education, or low educational attainment, were associated with no condom use at the time of their last sexual encounter and inconsistent condom use. Elsewhere on the continent, evidence suggests that the level of education is a key determinant of condom use [52]. In these studies, a positive association between educational level and condom use was attributed to higher educational attainment increases response to condom promotion. These observations highlight the need for creative strategies to increase the patterns of condom use among those with either no education or low educational attainment. Interventions may include the promotion of strategies that include community mobilisation and involvement of local organisations.
In addition, the findings revealed that risky alcohol consumption was associated with no condom use at last sexual encounter and inconsistent condom use. These observations highlight the need for public health interventions targeting both alcohol abuse and inconsistent condom use. Promoting consistent condom use as part of HIV risk-reduction interventions targeting high-risk drinkers is needed. Providing condoms in drinking venues could also be one of the important interventions to increase the level of consistent condom use in this population group [53].
Some studies have observed the association between ART and risky sexual behaviour after short durations on ART exposure [54,55,56]. However, others suggest the onset of changes in risky sexual behaviour over a much longer period after ART initiation [56]. These findings highlight the complexity of examining the association of ART and risky sexual behaviour and its variations among study participants in different settings. Therefore, it is important to continue to monitor risk reduction practices with the scaling up of the ART programme.
This study has some limitations. Sexual behaviours were self-reported and may be subject to recall bias and social desirability bias. In addition, the analysis cannot infer causality due to the cross-sectional nature of the study design, and is therefore limited only to assessing the associations between risky sexual behaviours, HIV status and exposure to ART. It is also important to note that the original data were collected nearly five years ago, and therefore the current situation may be different. Nevertheless, this study provides valuable information regarding risk compensation among HIV-positive youth and adults in South Africa.
5. Conclusions
Risk compensation is not apparent among HIV positive adults who are on ART regarding multiple sexual partnerships, condom use during their last sexual encounter and consistent condom use. Instead, our analysis revealed relatively less risky sexual behaviours among HIV positive individuals who were aware of their status and on ART, compared to HIV positive individuals who were aware of their status and not on ART. The study suggests the need to get all PLHIV on ARTs and interventions to reduce risky sexual behaviours in uninfected people to prevent HIV acquisition; with special efforts to reach the elderly, men, those with no education/low educational attainment and high-risk drinkers. Monitoring long-term trends in risky sexual behaviours among PLHIV after ART initiation remains a priority. Future studies should explore the role of type of sexual partnership/relationship and patterns of risky sexual behaviours among those on ART. In addition, the link between HIV positive status, ART and drivers of multiple sexual partnerships related to risk compensation need further research. Finally, more research is needed on the influence of sex partner characteristics (same age, older or younger) in relation to differences in the pattern of condom use among HIV-positive individuals.
Author Contributions
Conceptualization, N.Z.; methodology, K.Z. and M.M.; formal analysis and verification, M.M., S.J., E.M. and K.Z.; data curation, S.J., S.R. and K.Z.; writing—original draft preparation, N.Z.; writing—review and editing, N.Z., M.M., S.R., K.Z., L.S., O.S., S.M., E.M., E.I., S.S., P.M. and K.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval for the survey was granted by the Human Sciences Research Council (HSRC) Research Ethics Committee (REC) with protocol approval number REC: 4/18/11/15. Approval was also granted by the Associate Director for Science, Center for Global Health (CGH), Centers for Disease Control and Prevention (CDC).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data for this manuscript are openly available on the Human Sciences Research Council institutional repository available at https://repository.hsrc.ac.za/handle/20.500.11910/15468, Archive number: SABSSM 2017 Combined, URI: http://doi.org/10.14749/1585345902.
Conflicts of Interest
The authors declare no conflict of interest. The findings and conclusions of this report are those of the authors, and do not necessarily represent the official position of the funding agencies.
Funding Statement
This research was funded by President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) Cooperative Agreement Number NU2GGH001629.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simbayi L., Zuma K., Zungu N., Moyo S., Marinda E., Jooste S., Mabaso M., Ramlagan S., North A., Zyl J.V., et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017. HSRC Press; Cape Town, South Africa: 2019. [Google Scholar]
- 2.Jean K., Gabillard D., Moh R., Danel C., Fassassi R., du Loû A.D., Eholié S., Lert F., Anglaret X., Dray-Spira R. Effect of Early Antiretroviral Therapy on Sexual Behaviors and HIV-1 Transmission Risk Among Adults with Diverse Heterosexual Partnership Statuses in Cote d’Ivoire. J. Infect. Dis. 2014;209:431–440. doi: 10.1093/infdis/jit470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinsztejn B., Hosseinipour M.C., Ribaudo H.J., Swindells S., Eron J., Chen Y.Q., Wang L., Ou S.-S., Anderson M., McCauley M., et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: Results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect. Dis. 2014;14:281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen M.S., Chen Y.Q., McCauley M., Gamble T., Hosseinipour M.C., Kumarasamy N., Hakim J.G., Kumwenda J., Grinsztejn B., Pilotto J.H., et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goosby E., Von Zinkernagel D., Holmes C., Haroz D., Walsh T. Raising the bar: PEPFAR and new paradigms for global health. J. Acquir. Immune Defic. Syndr. 2012;60:S158–S162. doi: 10.1097/QAI.0b013e31825d057c. [DOI] [PubMed] [Google Scholar]
- 6.Goosby E. The President’s Emergency Plan for AIDS Relief: Marshalling all tools at our disposal toward an AIDS-free generation. Health Aff. (Millwood) 2012;31:1593–1598. doi: 10.1377/hlthaff.2012.0241. [DOI] [PubMed] [Google Scholar]
- 7.Beyrer C., Birx D.L., Bekker L.-G., Barré-Sinoussi F., Cahn P., Dybul M.R., Eholié S.P., Kavanagh M.M., Katabira E.T., Lundgren J., et al. The Vancouver Consensus: Antiretroviral medicines, medical evidence, and political will. Lancet. 2015;386:505–507. doi: 10.1016/S0140-6736(15)61458-1. [DOI] [PubMed] [Google Scholar]
- 8.Tanser F., Bärnighausen T., Grapsa E., Zaidi J., Newell M.L. High Coverage of ART Associated with Decline in Risk of HIV Acquisition in Rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesh K.K., Flanigan T.P., Mayer K.H. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS. 2011;25:1939–1949. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS Country Factsheets, South Africa. 2019. [(accessed on 23 February 2021)]. Available online: https://www.unaids.org/en/regionscountries/countries/southafrica.
- 11.National Department of Health . Annual Performance Plan 2018/19–2020/21. Department of Health; Pretoria, South Africa: 2018. [Google Scholar]
- 12.Westercamp N., Agot K., Jaoko W., Bailey R.C. Risk compensation following male circumcision: Results from a two-year prospective cohort study of recently circumcised and uncircumcised men in Nyanza Province, Kenya. AIDS Behav. 2014;18:1764–1775. doi: 10.1007/s10461-014-0846-4. [DOI] [PubMed] [Google Scholar]
- 13.Macphail C.L., Sayles J.N., Cunningham W., Newman P.A. Perceptions of sexual risk compensation following posttrial HIV vaccine uptake among young South Africans. Qual. Health Res. 2012;22:668–678. doi: 10.1177/1049732311431944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassell M.M., Halperin D.T., Shelton J.D., Stanton D. Risk compensation: The Achilles’ heel of innovations in HIV prevention? BMJ. 2006;332:605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton L.A., Kalichman S. Risk compensation in HIV prevention: Implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr. HIV/AIDS Rep. 2007;4:165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma M., Romas J.A. Theoretical Foundations of Health Education and Health Promotion. Jones & Bartlett Publishers; Boston, MA, USA: 2012. [Google Scholar]
- 17.Berhan A., Berhan Y. Is the Sexual Behaviour of HIV Patients on Antiretroviral therapy safe or risky in Sub-Saharan Africa? Meta-Analysis and Meta-Regression. AIDS Res. Ther. 2012;9:14. doi: 10.1186/1742-6405-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltzer K., Ramlagan S. Safer sexual behaviours after 1 year of antiretroviral treatment in KwaZulu-Natal, South Africa: A prospective cohort study. Sex Health. 2010;7:135–141. doi: 10.1071/SH09109. [DOI] [PubMed] [Google Scholar]
- 19.Risher K., Rehle T., Simbayi L., Shisana O., Celentano D.D. Antiretroviral treatment is not associated with risk compensation among HIV infected South Africans. AIDS Behav. 2016;20:710–716. doi: 10.1007/s10461-015-1125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apondi R., Bunnell R., Ekwaru J.P., Moore D., Bechange S., Khana K., King R., Campbell J., Tappero J., Mermin J. Sexual behavior and HIV transmission risk of Ugandan adults taking antiretroviral therapy: 3 year follow-up. AIDS. 2011;25:1317–1327. doi: 10.1097/QAD.0b013e328347f775. [DOI] [PubMed] [Google Scholar]
- 21.Shafer L.A., Nsubuga R.N., White R., Mayanja B.N., Chapman R., O’Brien K., Van der Paal L., Grosskurth H., Maher D. Antiretroviral therapy and sexual behavior in Uganda: A cohort study. AIDS. 2011;25:671–678. doi: 10.1097/QAD.0b013e328341fb18. [DOI] [PubMed] [Google Scholar]
- 22.Delva W., Helleringer S. Beyond Risk Compensation: Clusters of Antiretroviral Treatment (ART) Users in Sexual Networks Can Modify the Impact of ART on HIV Incidence. PLoS ONE. 2016;11:e0163159. doi: 10.1371/journal.pone.0163159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasse B., Ledergerber B., Hirschel B., Vernazza P., Glass T.R., Jeannin A., Evison J.M., Elzi L., Cavassini M., Bernasconi E., et al. Swiss HIV Cohort Study. Frequency and determinants of unprotected sex among HIV-infected persons: The Swiss HIV Cohort Study. Clin. Infect. Dis. 2010;51:1314–1322. doi: 10.1086/656809. [DOI] [PubMed] [Google Scholar]
- 24.Doyle J.S., Degenhardt L., Pedrana A.E., McBryde E.S., Guy R.J., Stoové M.A., Weaver E.R., Grulich A.E., Lo Y.-R., Hellard M.E. Effects of HIV antiretroviral therapy on sexual and injecting risk-taking behavior: A systematic review and meta-analysis. Clin. Infect. Dis. 2014;59:1483–1494. doi: 10.1093/cid/ciu602. [DOI] [PubMed] [Google Scholar]
- 25.Nkhoma K., Ahmed A., Alli Z., Sherr L., Harding R. Does sexual behaviour of people with HIV reflect antiretroviral therapy as a preventive strategy? A cross-sectional study among outpatients in Kenya. BMC Public Health. 2019;19:1254. doi: 10.1186/s12889-019-7581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye D.K., Kakaire O., Osinde M.O., Lule J.C., Kakande N. The impact of highly active antiretroviral therapy on high-risk behaviour of HIV-infected patients in sub-Saharan. Africa J. Infect. Dev. Ctries. 2013;7:436–447. doi: 10.3855/jidc.2644. [DOI] [PubMed] [Google Scholar]
- 27.Statistics South Africa . Mid-Year Population Estimates 2017. Statistics South Africa; Pretoria, South Africa: 2017. [Google Scholar]
- 28.Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the Alcohol Use Disorders Screening Test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 29.Morojele N.K., Nkosi S., Kekwaletswe C.T., Shuper P.A., Manda S.O., Myers B., Parry C.D.H. Utility of Brief Versions of the Alcohol Use Disorders Identification Test (AUDIT) to Identify Excessive Drinking Among Patients in HIV Care in South Africa. J. Stud. Alcohol Drugs. 2016;78:88–96. doi: 10.15288/jsad.2017.78.88. [DOI] [PubMed] [Google Scholar]
- 30.Coates T.J., Richter L., Caceres C. Behavioural strategies to reduce HIV transmission: How to make them work better. Lancet. 2008;372:669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzan-Monti M., Lorente N., Demoulin B., Marcellin F., Préau M., Dray-Spira R., Lert F., Spire B. Sexual risk behaviour among people living with HIV according to the biomedical risk of transmission: Results from the ANRS-VESPA2 survey. J. Int. AIDS Soc. 2016;19:20095. doi: 10.7448/IAS.19.1.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joint United Nations Programme on AIDS (UNAIDS) UNAIDS Global Report on the AIDS Epidemic Geneva, Switzerland, UNAIDS. 2010. [(accessed on 7 November 2019)]. Available online: http://search.unaids.org/search.asp?lg=en&search=2010%20report%20on%20the%20global%20aids%20epidemic.
- 33.Yi S., Tuot S., Yung K., Kim S., Chhea C., Saphonn V. Factors Associated with Risky Sexual Behavior among Unmarried Most-at-Risk Young People in Cambodia. Am. J. Public Health Res. 2014;5:211–220. doi: 10.12691/ajphr-2-5-5. [DOI] [Google Scholar]
- 34.Mishra V., Bignami-Van Assche S. Concurrent Sexual Partnership and HIV Infection: Evidence from National Population-Based Surveys, (UNAIDS 2009) Macro-International Inc.; Calverton, MD, USA: 2009. [Google Scholar]
- 35.Exavery A., Kanté A.M., Tani K., Hingora A., Phillips J.F. Socio-demographic drivers of multiple sexual partnerships among women in three rural districts of Tanzania. HIV/AIDS. 2015;7:105. doi: 10.2147/HIV.S76694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasrullah M., Oraka E., Chavez P.R., Johnson C.H., DiNenno E. Factors Associated with Condom Use Among Sexually Active U.S. Adults, National Survey of Family Growth, 2006–2010 and 2011–2013. J. Sex Med. 2017;14:541–550. doi: 10.1016/j.jsxm.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christopher H.R., Riley S.J., Richard L., Schell H., Patricia D. Variability in Condom Use Trends by Sexual Risk Behaviors: Findings from the 2003–2015 National Youth Risk Behavior Surveys. Sex. Transm. Dis. 2018;45:400–405. doi: 10.1097/OLQ.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kordoutis P., Loumakou M., Sarafidou J. Heterosexual relationship characteristics, condom use and safe sex practices. AIDS Care. 2001;12:767–782. doi: 10.1080/09540120020014318a. [DOI] [PubMed] [Google Scholar]
- 39.Kathleen Ford KSohn W., Lepkowski J. Characteristics of Adolescents’ Sexual Partners and Their association with use of Condoms and other contraceptive methods. Fam. Plan. Perspect. 2001;33:100–105. [PubMed] [Google Scholar]
- 40.Prata N., Vahidnia F., Fraser A. Gender and Relationship Differences in Condom Use Among 15–24-Year-Olds in Angola. Int. Perspect. Sex. Reprod. Health. 2005;31:192–199. doi: 10.1363/3119205. [DOI] [PubMed] [Google Scholar]
- 41.Woolf S.E., Maisto S.A. Gender Differences in Condom Use Behavior? The Role of Power and Partner-Type. Sex Roles. 2008;58:689–701. [Google Scholar]
- 42.Qiao J., Guo Y., Zhu Y., Hong Y.A., Xu Z., Zeng C., Zhang H., Cai W., Li L., Liu C., et al. Gender differences in the relationship of sexual partnership characteristics and inconsistent condom use among people living with HIV in China. AIDS Care. 2020;32:128–135. doi: 10.1080/09540121.2019.1622632. [DOI] [PubMed] [Google Scholar]
- 43.Ernestine A., Duncan W. Influence of Partner Type on Condom Use. J. Hum. Behav. Soc. Environ. 2011;21:784–802. [Google Scholar]
- 44.Yamamoto N., Ejima K., Nishiura H. Modelling the impact of correlations between condom use and sexual contact pattern on the dynamics of sexually transmitted infections. Theor. Biol. Med. Model. 2018;15:6. doi: 10.1186/s12976-018-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adetunji J. Condom Use in Marital and Nonmarital Relationships in Zimbabwe. Int. Perspect. Sex. Reprod. Health. 2000;26:196–200. doi: 10.2307/2648258. [DOI] [Google Scholar]
- 46.Eisele T., Mathews C., Chopra M., Lurie M.N., Brown L., Dewing S., Kendall C. Changes in risk behaviour among HIV-positive patients during their first year of antiretroviral therapy in Cape Town South Africa. AIDS Behav. 2008;13:1097–1105. doi: 10.1007/s10461-008-9473-2. [DOI] [PubMed] [Google Scholar]
- 47.Chimbindi N.Z., McGrth N., Herbst K., Tint K.S., Newell M.L. Socio-Demographic Determinants of Condom Use among Sexually Active Young Adults in Rural KwaZulu-Natal, South Africa. Open AIDS J. 2010;4:88–95. doi: 10.2174/1874613601004010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisele T.P., Mathews C., Chopra M., Brown L., Silvestre E., Daries V., Kendall C. High levels of risk behavior among people living with HIV Initiating and waiting to start antiretroviral therapy in Cape Town South Africa. AIDS Behav. 2008;12:570–577. doi: 10.1007/s10461-007-9279-7. [DOI] [PubMed] [Google Scholar]
- 49.Walusaga H.A., Kyohangirwe R., Wagner G.J. Gender differences in determinants of condom use among HIV clients in Uganda. AIDS Patient Care STDS. 2012;26:694–699. doi: 10.1089/apc.2012.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calsyn D.A., Peavy M., Wells E.A., Campbell A.N., Hatch-Maillette M.A., Greenfield S.F., Tross S. Differences between men and women in condom use, attitudes, and skills in substance abuse treatment seekers. Am. J. Addict. 2013;22:150–1577. doi: 10.1111/j.1521-0391.2013.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shai N.J., Jewkes R., Nduna M., Dunkle K. Masculinities and condom use patterns among young rural South Africa men: A cross-sectional baseline survey. BMC Public Health. 2012;12:462. doi: 10.1186/1471-2458-12-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagarde E., Carael M., Glynn J.R., Kanhonou L., Abega S., Kahindo M., Musonda R., Auvert B., Buve A. Education Level is Associated with Condom Use within Non-Spousal Partnerships in Four Cities of Sub-Saharan Africa. AIDS. 2001;15:399–1408. doi: 10.1097/00002030-200107270-00009. [DOI] [PubMed] [Google Scholar]
- 53.Beksinska M.E., Smit J.A., Mantell J.E. Progress and challenges to male and female condom use in South Africa. Sex Health. 2012;9:51–58. doi: 10.1071/SH11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diabaté S., Alary M., Koffi C.K. Short-term increase in unsafe sexual behaviour after initiation of HAART in Côte d’Ivoire. AIDS. 2008;22:154–156. doi: 10.1097/QAD.0b013e3282f029e8. [DOI] [PubMed] [Google Scholar]
- 55.Yaya I., Saka B., Landoh D.E., Patchali P.M., Makawa M.-S., Senanou S. Sexual risk behaviour among people living with HIV and AIDS on antiretroviral therapy at the regional hospital of Sokodé, Togo. BMC Public Health. 2014;14:636. doi: 10.1186/1471-2458-14-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okoboi S., Castelnuovo B., Moore D.M., Musaazi J., Kambugu A., Birungi J., Kaleebu P., Nanfuka M., Kamya M.R., Van Rie A. Risky sexual behavior among patients on long-term antiretroviral therapy: A prospective cohort study in urban and rural Uganda. AIDS Res. Ther. 2018;15:15. doi: 10.1186/s12981-018-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this manuscript are openly available on the Human Sciences Research Council institutional repository available at https://repository.hsrc.ac.za/handle/20.500.11910/15468, Archive number: SABSSM 2017 Combined, URI: http://doi.org/10.14749/1585345902.