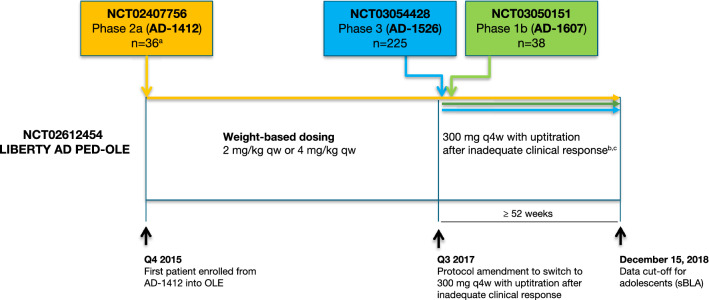
Fig. 1.
LIBERTY AD PED-OLE study schematic. a36 patients enrolled from R668-AD-1412 phase IIa study; five patients received only the weight-based dose regimen and were excluded, 31 patients from R668-AD-1412 were analyzed. bPatients enrolled from R668-AD-1412 switched from weight-based dosing to 300 mg q4w at the time of protocol amendment, with uptitration after inadequate response, whereas all patients enrolled from R668-AD-1526 and R668-AD-1607 started directly on the q4w or q2w regimen. cUptitration to 200 mg (body weight < 60 kg) or 300 mg (body weight ≥ 60 kg) q2w at week 16 upon inadequate clinical response, or prior to week 16 if deemed necessary by the investigator. AD atopic dermatitis, OLE open-label extension, qw weekly, q2w every 2 weeks, q4w every 4 weeks, sBLA supplemental Biologics License Application