Abstract
Background
Acute respiratory distress syndrome (ARDS) is still associated with significant mortality, especially the elderly and those with comorbidities are at highest risk of death. Neuromuscular blocking agents (NMBAs) are used in a large but highly variable proportion of patients with ARDS.
Case presentation
We describe the case of one critically ill patient with serious ARDS, because of virus pneumonia. In spite of the reduced tidal volume to 4–6 mL/kg of predicted body weight (PBW) and prone position were applied timely, the irresistible progress of disease leaded to an amazing prolonged application of deep sedation and analgesia, as well as NMBA, for the purpose of lung-protective mechanical ventilation.
Result
The clinical and biochemical parameters guided us toward the recognition that cisatracurium, bolus of 0.1 mg/kg followed by a median infusion rate of 1.91 (1.43–9.52) μg/kg.min, combined with continuous infusion of midazolam 3.43 (2.06–6.17) mg/kg.d and remifentanil 3.79 (3.43–8.57) μg/kg.h is efficacious and suitable for continuous muscle paralysis.
Conclusion
The intensive care unit (ICU)-acquired weakness (ICU-AW) was inevitable. Besides, an increased demand on drug concentration with the extension of medication time was observed as well.
Keywords: Acute respiratory distress syndrome (ARDS), Neuromuscular-blocking agents, Intensive care unit (ICU)-Acquired weakness (ICU-AW)
Highlights
-
•
The continuous infusion of cisatracurium is safety and efficacy.
-
•
The drug concentration of cisatracurium is time-dependent.
-
•
ICU-acquired weakness (ICU-AW) is inevitable for the critical illness.
1. Introduction and importance
Acute respiratory distress syndrome (ARDS), characterized by the acute onset of hypoxemia and bilateral pulmonary infiltrates with the exception of left heart failure [1], is present in 10% of patients admitted to an intensive care unit (ICU), and the hospital mortality is about 40% [2]. ARDS is one of the most severe conditions in critical illness and also one of the most challenging regarding the management of analgesia and sedation [3]. Neuromuscular blocking agents (NMBAs) are frequently used in the most severe forms of ARDS [2], when sedation alone is inadequate. Indeed, even deep sedation is often not enough to control minute ventilation, plateau pressure and tidal volume in the early phase of ARDS [4]. Moreover, the NMBAs can facilitate the patients for prone positioning, high positive end expiratory pressure (PEEP) levels and enable the tolerance of “permissive” hypercapnia [5].
The continuous administration of cisatracurium appears particularly adapted to such objectives, because of its more potent and less risk than atracurium [6]. In addition, after prolonged neuromuscular paralysis, the recovery of cisatracurium appears much more rapid than of vecuronium [7]. Despite the advantages mentioned above, the appalling protracted application of NMBAs in the present case still shocked us, and prompted us to review the literature about pharmacokinetics of cisatracurium in patients, especially those with ARDS. We first reported the efficacy of continuously prolonged infusion of cisatracurium besilate in patients with severe ARDS, at the same time, the muscle-related side effect, such as ICU-AW, cannot be ruled out in this case. In describing this case, we have an intent of highlighting how crucial it is to monitor any adverse events and how important of the general care for the patient.
2. Methods
Because this was a retrospective study and there was no design in the treatment process, especially the patient information was de-identified during subsequent data collection. So this case report was not registered in any clinical trial. Even so, the written informed consent was obtained from the patient's family for publication of this case report and we collected and reported this case in line with the SCARE 2020 criteria [8].
3. Case presentation
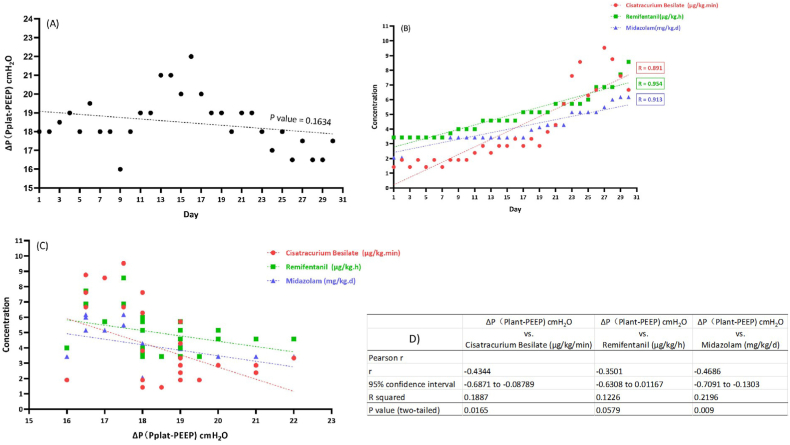
An 84-year-old male was admitted to our hospital because of dry pharynx with slight cough without the ambulance, and he was diagnosed with virus pneumonia soon on January 27, 2021. His absolute lymphocyte count was 0.62 × 109/L and leucocyte count was 3.35 × 109/L at admission with no obvious fever, neither shortness of breath nor chest pain. The chest X-ray and CT at admission and his medical history were shown in Fig. 1 and Table 1. The patient employed the high flow humidified oxygen delivery device with the assistance of prone position on the second day, because of the rapid deterioration of clinical symptoms and laboratory indexes (PaO2/FiO2 ratio decreased from 257 to 165 mmHg). Unfortunately, accompanied by the irresistible progresses of the disease as shown in Fig. 2, rescues of mechanical ventilation with sufficient analgesia and sedation were applied on February 05, 2021. Due to the serious man-machine confrontation and the urgent need for prone position, when the PaO2/FiO2 ratio <150 mmHg as the guideline recommended [9], cisatracurium besilate was injected with 0.1 mg/kg to paralyze the muscles on February 08, 2021. Then maintain a continuous infusion of cisatracurium to control the platform pressure under 30 cmH2O [10]. And an additional bolus of the drug was administered when plateau airway pressure exceeded 32 cmH2O to prevent ventilator-associated lung injury [11]. From Fig. 3(A), the driving pressure (ΔP = Pplat - PEEP) of our patient was controlled ideally during the whole course. Because of the predictable long-term intubation, the percutaneous tracheotomy was performed three days after mechanical ventilation. The patient developed ICU-AW during the treatment proved by the two-dimensional ultrasound (Fig. 4). Besides, the patient developed deep vein thrombosis (DVT) twice also conformed by the ultrasound features, fortunately, the clot disappeared with the use of low molecular weight heparin (LMWH) and the pulmonary embolism (PE) was excluded through CT pulmonary angiography. Till the end of collection, the median continuous infusion rate of cisatracurium was 1.91 (1.43–9.52) μg/kg.min for more than 720 hours. Unfortunately, despite a long period of positive complex therapy, the patient still deteriorated to the pulmonary fibrosis and was difficult to wean the deep sedation, analgesia and muscle relaxation aiming at control the platform pressure (<30 cmH2O). Due to the advanced age, the severity and complexity of the patient's disease, the patient eventually died eight months after his admission.
Fig. 1.
The computed tomographic scan of our patient with ARDS at admission.
Table 1.
Demographic data of the patient at admission.
| Sex | Male |
|---|---|
| Age (y) | 84s |
| Actual weight (kg) | 70 |
| Predicted body weight (kg) | 67 |
| Height (cm) | 172 |
| BMI | 23.67 |
| Blood type | B |
| Coexisting chronic diseases | Hypertension |
| Cerebral infarction | |
| Medication use | Amlodipine |
Fig. 2.
The computed tomographic scan of our patient with ARDS three days after admission.
Fig. 3.
(A) The driving pressure (ΔP = Pplat - PEEP) of our patient was controlled ideally in the whole course. (B) The concentration of cisatracurium, midazolam and remifentanil respectly, was time-dependent significantly (P < 0.05). (C) The driving pressure (ΔP) was related to the concentration of analgesics, sedatives and muscle relaxant. (D) The statistical analysis results of Fig. 3(C).
Fig. 4.
The muscle cross-sectional area and layer thickness detected by ultrasound. The ultrasound measurement of cross-sectional area of rectus femoris muscle (A, C) and thickness of vastus intermedius muscle (B, D) on the first day with cisatracurium and one week later, respectively.
4. Discussion
NMBAs are suggested to administer by continuous intravenous infusion in the early course of ARDS patients with a PaO2/FiO2 less than 150 mmHg [11]. The major advantages of NMBAs are limiting the asynchronies and plateau pressure, increasing the chest compliance, which can decrease the baro- and volutrauma, thereby to optimize gas exchange [12]. Among these agents, cisatracurium besilate, a non-depolarizing NMBA has been extensively studied and widely used in clinical practice. The in vitro and in vivo data suggest that the predominant elimination of cisatracurium besilate is pH- and temperature-dependent chemical (or Hofmann) degradation, occurs in the blood plasma and extracellular fluid (accounted for 76.9%) [13], does not depend on renal or hepatic function, which may increase its use in patients with multiorgan dysfunction [14] or the elderly patients [15].
In patients with severe ARDS, early administration of a neuromuscular blocking agent can improve the 90-day survival without increasing the muscle weakness [16]. However, in the most of literatures, the NMBAs are commonly used for a short period with ARDS, such as 48 hours [16,17], which was very different from our patient. Because of the recovery from neuromuscular block was independent of cisatracurium dose over the range 0.1–0.4 mg/kg [18], we chosen 0.1 mg/kg injected to avoid unnecessary drug exposure. Different from elective surgery, ICU is a complex environment with many drug interactions (e.g. anesthetics, antibiotics, antiarrhythmic drugs, diuretics, etc) and other conditions (e.g. hypothermia, electrolyte imbalance, acidosis, fever, etc) that can potentiate or weaken the neuromuscular blocking activity [13]. The popular method to monitor the dose-response to an NMBA is train-of-four stimulation (TOF). However, because the results of critically ill patients are inconsistent [11], the current guidelines recommend TOF monitoring only as an adjunct to clinical assessment when monitoring NMBA and do not provide guidance for dosing or titration [19]. On the other hand, the Richmond Agitation-Sedation Scale (RASS) should use to adapt sedative requirements prior to starting NMBAs [12]. We used a continuous infusion of midazolam 3.43 (2.06–6.17) mg/kg.d and remifentanil 3.79 (3.43–8.57) μg/kg.h to achieve a RASS inferior to −4 [3,11,12]. Because the variability in patient response and the confounding influence of electromyography activity, the utility of the processed electroencephalogram signal (such as bispectral index score, BIS) as a reliable monitor of sedation in critically ill patients is reduced, especially when combined with muscle relaxants [11,20]. Based on all the facts above, the real-time and dynamic clinical assessment by clinicians at the bedside of patients is the most important. On the contrary, the necessary monitoring is still needed, which can provide some objective diagnostic information for clinicians.
The median continuous infusion rate of cisatracurium of our patient was 1.91 (1.43–9.52) μg/kg.min as displayed in Table 2, which was significantly lower than previous studies [[4], [5], [6], [7]]. Nevertheless, the prolonged infusion still raise our concern for the tolerance and any adverse events. Hypersensitivity and resistance to NMBAs, due to either an increase or a decrease in the number or sensitivity of receptors, are observed in a number of clinical states [11]. In our case, even though the infusion rate was significantly lower than other previous studies [[4], [5], [6], [7]], with more than 720 hours, the dosage of cisatracurium, midazolam and remifentanil respect, was time-dependent significantly (P < 0.05) as shown in Fig. 3(B). By further analysis displayed in Fig. 3(C-D), we found the driving pressure (ΔP) was related to the dosage of analgesics, sedatives and muscle relaxant, which was easy to understand. Thus, the growing demand for continuous infusion of analgesics, sedatives and NMBA perhaps due to the progress of disease, and on the other hand, maybe ascribe to the drugs tolerance. This topic is also the focus and interest of our future research.
Table 2.
Mechanical ventilation (MV) parameters and clinical information associated with the usage of cisatracurium.
| Intervals between initiation of MV and muscle relaxation | 48 hours |
|---|---|
| APACHE II scores (24 h after NMB drug administration) | 26 |
| PaO2/FiO2 ratio median (IQR) | 147 (117–307) mmHg |
| Vt median (IQR) | 370 (350–460) mL |
| PaO2 median (IQR) | 81 (69–177) mmHg |
| PaCO2 median (IQR) | 43 (34.9–61.7) mmHg |
| PetCO2 median (IQR) | 33 (28–48) mmHg |
| Respiratory Rate (RR) median (IQR) | 22 (18-26) |
| Pplat median (IQR) | 24 (23–29) cmH2O |
| PEEP median (IQR) | 6 (5–8) cmH2O |
| FiO2 median (IQR) | 50 (45–70) % |
| Blood Glucose (arterial blood) median (IQR) | 7.8 (5–15.5) mmol/L |
| Midazolam, median (IQR) | 3.43 (2.06–6.17) mg/kg.d |
| Remifentanil, median (IQR) | 3.79 (3.43–8.57) μg/kg.h |
| Cisatracurium Besilate, median (IQR) | 1.91 (1.43–9.52) μg/kg.min |
| Data collected from February 8th, 2021 to March 9th, 2021 | |
| IQR: interquartile range | |
ICU-acquired weakness (ICU-AW), which refers to a group of neuromuscular disorders, is a frequent complication of critical illness with the morbidity about 30%–50% [21]. It generally affects the limb muscles, the diaphragm muscle, and even the global respiratory muscles [22]. As we known, prolonged administration of neuromuscular blocking agents is particularly associated with subsequent neuromuscular weakness [17]. The generally accepted standard for diagnosis of ICU-AW is through strength assessment using six points Medical Research Council (MRC) score [23]. However, strength assessment needs patients to be conscious, attentive and able to cooperative during testing, which is challenging for many critically ill patients, due to serious illness status, mechanical ventilation, and the use of anesthetic medications [24]. Additionally, electrophysiological recordings or muscle biopsy are not routinely carried out in the majority of ICU [24]. Muscle ultrasound is a convenient approach to investigate the muscle changes, the most common imaging features are reduction tendency of the cross-sectional area or decrescent muscle thickness [25], decrescent pennation angle or increase in echo intensity [26]. Despite the enteral nutrition was given as early as possible according to the guidelines [27], and the patient's metabolic markers, such as prealbumin and albumin improved significantly as shown in Table 3, the patient's muscle atrophy was pretty obviously (Fig. 4). Hyperglycemia is another potential driving force in the onset of ICU-AW [21]. So we strictly controlled the blood glucose, synthetically by arterial blood (given more weight) and capillary blood, less than 180 mg/dL according to suggestions drawn from previous studies [11,28]. Yet there still is a question as to whether the patients receiving sustained NMBA infusions require special nutritional considerations [11]? It proved that the gut absorption and gastric emptying were unaffected with NMBAs use [11]. Therefore, evaluating the underlying critical illness, such as prolonged immobility, opioid use, fluid imbalances and the potential infection will guide the clinician in determining whether the patient has a functional gastrointestinal tract independent of whether or not an NMBA is used.
Table 3.
Biochemical outcomes: comparison between neat and infusion of cisatracurium.
| Variables | neat (mean ± SD) | continuous infusion of cisatracurium (mean ± SD) | P |
|---|---|---|---|
| Prealbumin (mg/L) | 112.18 ± 30.48 | 214.3 ± 75.49 | <0.05 |
| Cr (μmol/L) | 57.31 ± 12.06 | 55.79 ± 8.59 | 0.66 |
| BUN (mmol/L) | 8.10 ± 1.59 | 12.34 ± 3.58 | <0.05 |
| Albumin (g/L) | 34.82 ± 1.66 | 37.378 ± 3.32 | <0.05 |
| WBC (10^9/L) | 12.55 ± 3.69 | 11.66 ± 3.16 | 0.45 |
| Neutrophil count (10^9/L) | 11.03 ± 3.37 | 10.26 ± 3.18 | 0.5 |
| Lymphocyte count (10^9/L) | 0.54 ± 0.25 | 0.75 ± 0.39 | 0.12 |
| Hb (g/L) | 148.82 ± 11.30 | 100.90 ± 11.46 | 1.48 |
| ALT (U/L) | 17.91 ± 2.47 | 40.86 ± 27.30 | <0.05 |
| IL-6 (pg/ml) | 33.59 ± 23.51 | 13.82 ± 29.29 | 0.17 |
| CD4 (cell/ul) | 158.83 ± 74.01 | 302.97 ± 185.04 | 0.2 |
| CD8 (cell/ul) | 94.67 ± 14.18 | 158.40 ± 82.34 | 0.2 |
| PLT (10^9/L) | 217.18 ± 51.29 | 288.23 ± 118.37 | 0.05 |
| APTT (second) | 36.31 ± 2.28 | 36.99 ± 4.84 | 0.66 |
| D-D (μg/ml) | 1.69 ± 0.73 | 1.40 ± 0.63 | 0.22 |
Data collected from January 28th, 2021 to March 9th, 2021 and the cisatracurium initiated on February 8th, 2021.
With the long-term usage of muscle relaxant, the obviously adverse reaction of the patient is bradycardia, though the plasma concentrations of metabolite laudanosine are low with cisatracurium than those seen with atracurium [29]. Of course, the renal function and the side-effect of midazolam and remifentanil may play a synergistic role with cisatracurium on bradycardia, which warning clinicians to take emergency measures on this cardiovascular effect. Another important topic is venous thromboembolism (VTE). Even the mechanical prophylaxis and anticoagulant drugs applied for the patient at his admission and throughout the course of disease, he still developed DVT for twice. The molecular mechanisms implicated in this thrombotic state appears involving infected endothelial cells, leukocytes and platelets, as well as complement activation and the hypoxic milieu produced by the virus which can further enhance these processes [30]. In addition, invasive operation, drug-interaction and the drug damage to vascular endothelium complicated the formation of thrombus.
Other important aspects including bleeding and coagulation, (potentially) infection control, liquid management, rewarming rate and safeguards to avoid unplanned extubation (UE), pressure ulcer management, etc, are all vital issues should be discussed daily by our medical staffs for the critically ill patients. However, possibly due to the older age and the critically compromised general condition of the patient, despite a long period of positive complex therapy, the patient still deteriorated to the pulmonary fibrosis and was difficult to wean the deep sedation, analgesia and muscle relaxation and the poor prognosis as you can imagine.
5. Conclusion
We presented the case of an elderly patient with continuously protracted infusion of cisatracurium besilate secondary to ARDS. The main clinical challenge related to the case was to control the platform pressure under 30 cmH2O with continuously infusion of muscle relaxants for more than 720 hours, which is a great challenge for clinicians and clinical nursing. In the process of treatment, the definition of intensive care is reflected everywhere. Finally, the clinical and biochemical parameters guided us toward the recognition that cisatracurium is efficacious and suitable for continuous muscle paralysis, despite the drug concentration is time-dependent. Meanwhile, the emergence of muscle-related side effect, such as ICU-AW cannot be ruled out in this case. Although there's still some issues worth discussing, this was the first case reported the astoundingly long period of administration of muscle relaxants.
6. Limitation of study
The limitations of this study were, the first, although the staff did their best, there are still inadequacies in the treatment process due to the defects of a single center. Second, the single sample is not universal, it may affect the conclusion.
Ethics approval and consent
Written informed consent was obtained from the patient's family for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
The authors declared that this study has received no financial support.
Authors’ contributions
All authors contributed to the inception, design, analysis, interpretation, and drafting of the research manuscript. Also, all authors read and approved the revised manuscript for publication. More specifically, Ziming Yuan sponsored this concept and was responsible for writing. Lei Pan collected data and Yang Wang interpreted data. Wei Wang was responsible for final review and modification.
Registration of research studies
Because this was a retrospective study and there was no design in the treatment process, especially the patient information was de-identified during subsequent data collection. So this case report was not registered in any clinical trial.
Guarantor
Ziming Yuan.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
Authors of this article have no conflict or competing interests. All of the authors approved the final version of the manuscript.
Acknowledgments
The authors would like to thank all the doctors and nurses of the Department of Critical Care Medicine as well special thanks to family of the patient for accepting and taking consent to conduct this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103718.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Brodie D., Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011;365:1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Chanques G., Constantin J.M., Devlin J.W., et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46:2342–2356. doi: 10.1007/s00134-020-06307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hraiech S., Forel J.M., Papazian L. The role of neuromuscular blockers in ARDS: benefits and risks. Curr. Opin. Crit. Care. 2012;18:495–502. doi: 10.1097/MCC.0b013e328357efe1. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky A.S. Neuromuscular blocking agents in ARDS. N. Engl. J. Med. 2010;363:1176–1180. doi: 10.1056/NEJMe1007136. [DOI] [PubMed] [Google Scholar]
- 6.Szakmany T., Woodhouse T. Use of cisatracurium in critical care: a review of the literature. Minerva Anestesiol. 2015;81:450–460. [PubMed] [Google Scholar]
- 7.Dhonneur G., Cerf C., Lagneau F., et al. The pharmacokinetics of cisatracurium in patients with acute respiratory distress syndrome. Anesth. Analg. 2001;93:400–404. doi: 10.1097/00000539-200108000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Papazian L., Aubron C., Brochard L., et al. Formal guidelines: management of acute respiratory distress syndrome. Ann. Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 11.Murray M.J., DeBlock H., Erstad B., et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit. Care Med. 2016;44:2079–2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 12.Hraiech S., Forel J.M., Guervilly C., et al. How to reduce cisatracurium consumption in ARDS patients: the TOF-ARDS study. Ann. Intensive Care. 2017;7:79. doi: 10.1186/s13613-017-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryson H.M., Faulds D. Cisatracurium besilate. A review of its pharmacology and clinical potential in anaesthetic practice. Drugs. 1997;53:848–866. doi: 10.2165/00003495-199753050-00012. [DOI] [PubMed] [Google Scholar]
- 14.Sottile P.D., Kiser T.H., Burnham E.L., et al. An observational study of the efficacy of cisatracurium compared with vecuronium in patients with or at risk for acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2018;197:897–904. doi: 10.1164/rccm.201706-1132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.H., Lee Y.C., Lee S.I., et al. Effective doses of cisatracurium in the adult and the elderly. Korean J Anesthesiol. 2016;69:453–459. doi: 10.4097/kjae.2016.69.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papazian L., Forel J.M., Gacouin A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 17.Moss M., Huang D.T., Brower R.G., et al. Early Neuromuscular blockade in the acute respiratory distress syndrome. N. Engl. J. Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belmont M.R., Lien C.A., Quessy S., et al. The clinical neuromuscular pharmacology of 51W89 in patients receiving nitrous oxide/opioid/barbiturate anesthesia. Anesthesiology. 1995;82:1139–1145. doi: 10.1097/00000542-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Torbic H., Bauer S.R., Personett H.A., et al. Perceived safety and efficacy of neuromuscular blockers for acute respiratory distress syndrome among medical intensive care unit practitioners: a multicenter survey. J. Crit. Care. 2017;38:278–283. doi: 10.1016/j.jcrc.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Aho A.J., Lyytikäinen L.P., Yli-Hankala A., et al. Explaining Entropy responses after a noxious stimulus, with or without neuromuscular blocking agents, by means of the raw electroencephalographic and electromyographic characteristics. Br. J. Anaesth. 2011;106:69–76. doi: 10.1093/bja/aeq300. [DOI] [PubMed] [Google Scholar]
- 21.Cheung K., Rathbone A., Melanson M., et al. Pathophysiology and management of critical illness polyneuropathy and myopathy. J. Appl. Physiol. 2021;130:1479–1489. doi: 10.1152/japplphysiol.00019.2021. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medrinal C., Combret Y., Hilfiker R., et al. ICU outcomes can be predicted by non invasive muscle evaluation: a meta-analysis. Eur. Respir. J. 2020;56:1902482. doi: 10.1183/13993003.02482-2019. [DOI] [PubMed] [Google Scholar]
- 23.Fan E., Cheek F., Chlan L., et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am. J. Respir. Crit. Care Med. 2014;190:1437–1446. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Wu J., Gu Q., et al. Changes in muscle ultrasound for the diagnosis of intensive care unit acquired weakness in critically ill patients. Sci. Rep. 2021;11:18280. doi: 10.1038/s41598-021-97680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puthucheary Z.A., McNelly A.S., Rawal J., et al. Rectus femoris cross-sectional area and muscle layer thickness: comparative markers of muscle wasting and weakness. Am. J. Respir. Crit. Care Med. 2017;195:136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parry S.M., El-Ansary D., Cartwright M.S., et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care. 2015;30 doi: 10.1016/j.jcrc.2015.05.024. 1151.e9-14. [DOI] [PubMed] [Google Scholar]
- 27.Singer P., Blaser A.R., Berger M.M., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Jacobi J., Bircher N., Krinsley J., et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit. Care Med. 2012;40:3251–3276. doi: 10.1097/CCM.0b013e3182653269. [DOI] [PubMed] [Google Scholar]
- 29.Moore L., Kramer C.J., Delcoix-Lopes S., et al. Comparison of cisatracurium versus atracurium in early ARDS. Respir. Care. 2017;62:947–952. doi: 10.4187/respcare.05102. [DOI] [PubMed] [Google Scholar]
- 30.Manolis A.S., Manolis T.A., Manolis A.A., et al. COVID-19 infection: viral macro- and micro-vascular coagulopathy and thromboembolism/prophylactic and therapeutic management. J. Cardiovasc. Pharmacol. Therapeut. 2021;26:12–24. doi: 10.1177/1074248420958973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.