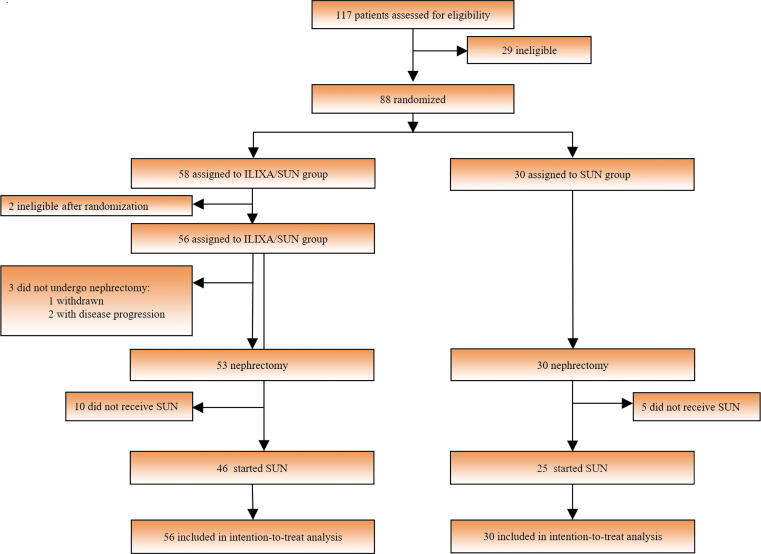
Fig. 1.
Overall trial profile. During enrollment, 111 patients were assessed for eligibility; 29 patients were screening failures and 88 were randomized. Patients were stratified according to the Heng criteria (high and intermediate risk). Fifty-eight patients (17 high-risk and 41 intermediate-risk patients) were allocated to receive ilixadencel (ILIXA) before nephrectomy and sunitinib (SUN) after nephrectomy (defined as the ILIXA/SUN group), whereas 30 patients (eight high-risk and 22 intermediate-risk patients) were allocated to sunitinib (SUN) alone after nephrectomy (defined as the SUN group).