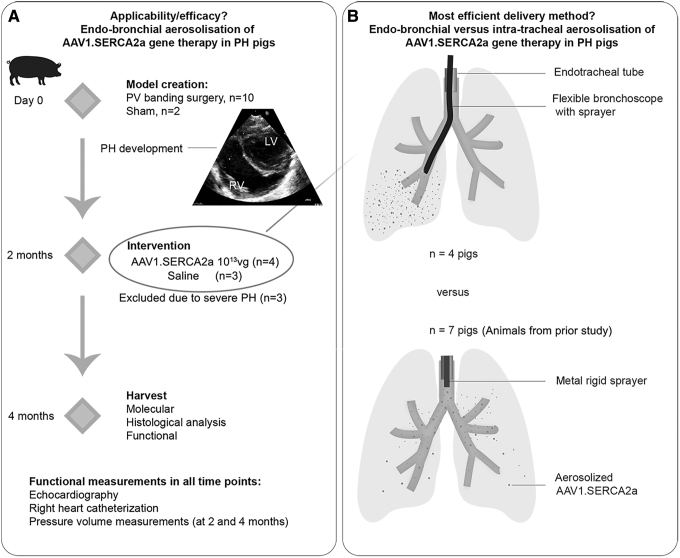
Figure 1.
Study design. (A) The part 1 of the study aimed to assess the applicability, efficacy, and safety of endobronchial aerosolization of AAV1.SERCA2a. PH was surgically induced by PV banding in 10–15 kg pigs. Two months later, the animals were randomized based on the AAV1 neutralizing antibody titer: animals with low antibody titer were enrolled in the SERCA2a therapy arm (AAV1.SERCA2a, n = 4), while pigs with high antibody titer received saline (n = 3). The delivery was done using a flexible bronchoscope and a sprayer for aerosolization. Two months after injection, the animals underwent functional evaluation and tissues were harvested. One pig died 1 month after AAV1.SERCA2a therapy likely due to nontherapy-related issue and was excluded from the functional analysis. Echocardiography and right heart catheterization were performed at all 3 time points. Pressure-volume loops were acquired at the 4-month time point. (B) The part 2 of the study was aimed at assessing the most efficient delivery mode for in-lung gene therapy. The lung tissues from animals in the prior study,11 which received intratracheal AAV1.SERCA2a, were used (n = 7). Other than the delivery method, the same experimental protocol was used. AAV1.SERCA2a was aerosolized intratracheally using a metal sprayer, advanced through the endotracheal tube. The stiff metal composition of the sprayer precluded further advancements to bronchi. AAV, adeno-associated viral; PH, pulmonary hypertension; PV, pulmonary vein.