Figure 5.
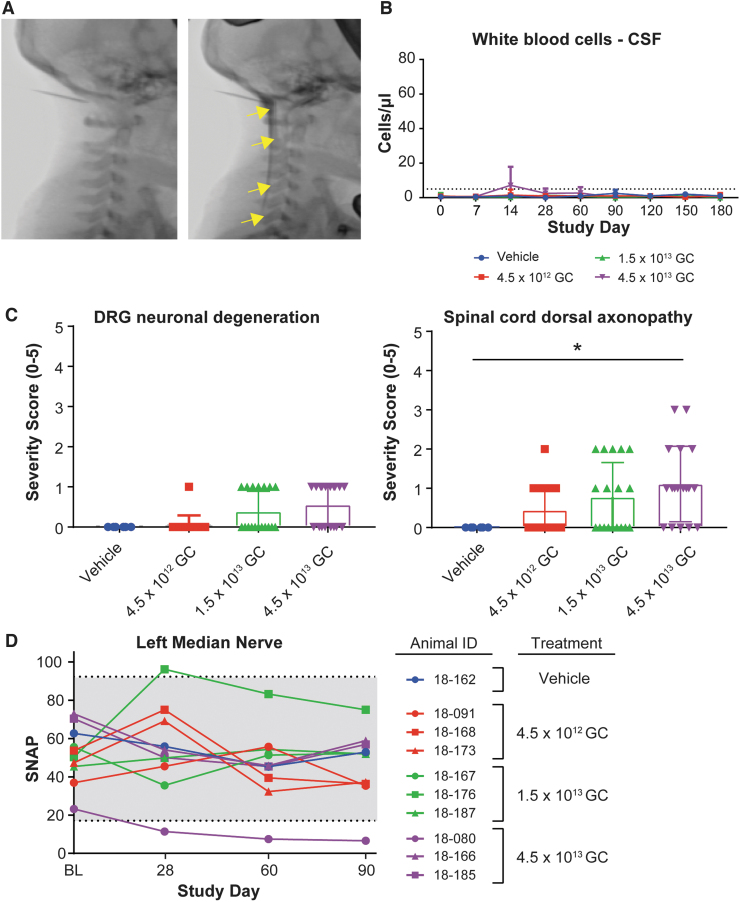
GLP toxicology study in rhesus macaques, safety. (A) Fluoroscopic images of the neck region, before and after contrast ICM administration, in juvenile rhesus macaques (animal 18–167, mid-dose group). Contrast injection was used to confirm needle placement before AAV administration. Arrows show the diffusion of the contrast in the cisterna magna and spinal canal. (B) WBC counts in the CSF of juvenile rhesus macaques treated ICM with artificial CSF (vehicle, n = 2) or AAVhu68.CB7.hGALCco.rBG at the dose of 4.5 × 1012 GC (low dose, n = 6), 1.5 × 1013 GC (mid dose, n = 6), or 4.5 × 1013 (high dose, n = 6). Half of the animals were euthanized for tissue collection 3 months postdosing; the other half were euthanized 6 months postdosing. Dotted line represents the upper limit of normal (C) Histopathological analysis, DRG neuronal degeneration, and spinal cord dorsal column axonopathy severity grades (0 = normal, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked, and 5 = severe). Three and six months cohorts are included, each data point represents 1 segment per animal (cervical, thoracic, and lumbar). *p < 0.05 Mann-Whitney rank test, alpha = 0.05. (D) Median nerve SNAP amplitudes at BL (before dosing), 28-, 60-, and 90-day postdosing in the 3 months cohorts. BL, baseline; DRG, dorsal root ganglia; GLP, Good Laboratory Practice; SNAP, sensory nerve action potential; WBC, white blood cell.