Abstract
The left ventricular assist device (LVAD) is one of the alternative treatments for heart failure (HF) patients. However, LVAD support is followed by thrombosis, and bleeding complications which are caused by high non-physiologic shear stress and antithrombotic/anticoagulant therapy. A high risk of complications occurs in the presence of the genotype polymorphisms which are involved in the coagulation system, hemostasis function and in the metabolism of the therapy. The aim of the study was to investigate the influence of single-nucleotide polymorphisms (SNP) in HF patients with LVAD complications. We analyzed 21 SNPs in HF patients (n = 98) with/without complications, and healthy controls (n = 95). SNPs rs9934438; rs9923231 in VKORC1, rs5918 in ITGB3 and rs2070959 in UGT1A6 demonstrated significant association with HF patients’ complications (OR (95% CI): 3.96 (1.42–11.02), p = 0.0057), (OR (95% CI): 3.55 (1.28–9.86), p = 0.011), (OR (95% CI): 5.37 (1.79–16.16), p = 0.0056) and OR (95% CI): 4.40 (1.06–18.20), p = 0.044]. Genotype polymorphisms could help to predict complications at pre- and post-LVAD implantation period, which will reduce mortality rate. Our research showed that patients can receive treatment with warfarin and aspirin with a personalized dosage and LVAD complications can be predicted by reference to their genotype polymorphisms in VKORC1, ITGB3 and UGT1A6 genes.
Keywords: genotype, polymorphism, heart failure, left ventricular assist device (LVAD), personalized medicine, thrombosis, bleeding
1. Introduction
Implantation of a left ventricular assist device (LVAD) is one of the best alternative treatments for end-stage heart failure (HF) patients, instead of heart transplantation (HT) [1]. After LVAD implantation patients experience improvements in their quality of life with reduced mortality. The survival rate after HT is 86%, while survival rate after LVAD implantation is 80%, which represents an optimal outcome [2]. In addition, LVAD implantation is better choice considering the acute shortage of the heart donors. Patients on the waiting list for HT have an increased risk of mortality rate compared to those patients who receive LVAD [3].
LVAD is implanted as a bridge-to-transplantation (BTT) or a destination therapy (DT). BTT is prescribed as a temporary treatment for patients who are heart donor candidates. DT is implanted as a lifetime support for patients who are not able to have HT because of their age, medical indications and complications [2,4,5,6].
However, LVAD is followed by complications which cause harm to quality of life in the first year after implantation. Bleeding and thrombosis are the most common complications, which require re-hospitalization [2,7,8,9,10]. Fifty percent of the mortality is caused by pump thrombosis and stroke. Consequently, patients require pump exchange or HT [8]. Anticoagulation therapy such as warfarin or aspirin is usually prescribed to reduce the risk of thromboembolic complications and pump thrombosis. However, a high dose of anticoagulation therapy predisposes patients to bleeding risks with a mortality rate of 9–10% [2,8,11,12]. Nowadays, complications can be reduced by genotype-guided warfarin therapy according to the genotyping test results for genetic polymorphisms of VKORC1 (vitamin K epoxide reductase complex 1) and CYP2C9 (cytochrome P450 2C9) genes. These genes are involved in warfarin metabolism and action [9,11,13]. Genome-wide association studies (GWAS) have shown that VKORC1 and CYP2C9 genes demonstrated 30% variability after a warfarin dose in European and Asian populations in non-LVAD patients [13,14,15]. Research on HF patients with implanted LVAD showed a difference in genotype frequency compared to other races such as European–American and African–American. Frequency of mutant variants for CYP2C9 and VKORC1 genes were significantly higher in European–Americans (38.0% and 50%, p < 0.05) than in African–Americans (9.7% and 3.2%, p < 0.05) [9]. There were no other investigations on GWAS in HF patients with implanted LVAD and their associations with complications [9,11,13].
One common reason for the blood clotting and bleeding events is platelet dysfunction [16]. Platelet dysfunctions are caused by the high non-physiologic shear stress (NPSS) at the blade region of the LVAD’s rotary. Shear stress induces platelet receptor damage and shedding. There are three important adhesive glycoprotein receptors on the membrane of the platelet: GPIbα, GPVI, and GPIIb/IIIa. Platelet activation, aggregation and adhesion processes occur when glycoprotein receptors GPIbα bind to von Willebrand factor (VWF), GPVI binds to collagen, and the GPIIb/IIIa to fibrinogen binds to VWF [17].
Apart from the shear-stresses, patient’s genetic information also influences the development of LVAD complications. Consequently, the purpose of our research is to study the influence of single-nucleotide polymorphisms (SNP) of the genes encoding blood coagulation processes, the metabolism of the anticoagulant, on antithrombotic therapy and the impact on complications development after LVAD implantation in HF patients.
2. Materials and Methods
2.1. Study Participants
The study was conducted in accordance with the Declaration of Helsinki. Research protocol was approved by the Ethics Committees of the National Laboratory Astana, Nazarbayev University (No.16 from 11 March 2015) and the National Research Cardiac Surgery Center (NRCC), Nur-Sultan, Kazakhstan (No.16 from 24 April 2015). Written informed consent was obtained from all participants. During the data collection, all personal information on patients is encoded and data depersonalized, to protect the rights of patients and not disclose their personal information. Written informed consent has been obtained from the patient(s) to publish this paper.
We recruited consecutively 100 HF patients with implanted LVAD during 2011–2016 when they were implanted with LVAD as BTT and DT at the NRCC. Two patients under 18 years old (9 and 16 years old) were not included in the analysis. HF patients (age ≥ 18) were diagnosed with ischemic cardiomyopathy (ICM), dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM) and valvular heart disease (VHD) by the cardiologists at NRCC. New York Heart Association (NYHA) functional class IIIA was noted in 34 patients (34.7%), class IIIB in 34 patients (34.7%) and class IV in 26 patients (26.5%) before LVAD implantation (Table 1). Ninety-seven (99%) patients were suffering from heart failure reduced ejection fraction (HFrEF). There were three types of LVADs implanted: 18 (18.4%) HeartWare HVADs (HW) (HeartWare Inc., Framingham MA, USA), 34 (34.7%) HeartMate II (HM2) (Thoratec Corporation, Pleasanton, CA, USA) and 46 (46.9%) HeartMate III (HM3) (St Jude Medical, Huntingdon, Cambridgeshire, UK) (Table 1). Patients had three-years’ follow up from 2014 till 2017 with a median of 18 months. Venous blood samples were obtained in sterile vacutainers with K2EDTA for further genetic analysis.
Table 1.
Baseline demographic characteristics of Control group and HF patients.
| Characteristic | Control Group, N = 95 | HF Patients, N = 98 | p Value |
|---|---|---|---|
| Age (years) | 44.01 ± 13.8 | 52.7 ± 11.0 | 0.001 ** |
| Gender | |||
| Male | 63 (66.3) | 92 (93.9) | 0.001 |
| Female | 32 (33.7) | 6 (6.1) | |
| Ethnicity | |||
| Asian | 60 (63.2) | 77 (78.6) | 0.03 |
| Caucasian | 35 (36.8) | 21 (21.4) | |
| Body weight (kg) | 69.5 ± 14.0 | 79.8 ± 13.9 | 0.001 ** |
| Height (cm) | 168.3 ± 7.46 | 169.8 ± 6.36 | 0.15 * |
| BMI (kg/m) | 24.6 ± 4.9 | 27.7 ± 4.5 | 0.001 ** |
| SBP | 114.4 ± 9.9 | 104.8 ± 15.5 | 0.001 ** |
| DBP | 75.7 ± 5.7 | 71.2 ± 10.3 | 0.001 ** |
| History of smoking | |||
| Smokers | 38 (40.0) | 58 (59.2) | 0.01 |
| Non-smokers | 57 (60.0) | 40 (40.8) | |
| Diagnosis | - | ||
| ICM | - | 44 (44.9) | |
| DCM | - | 40 (40.8) | |
| HCM | - | 11 (11.2) | |
| VHD | - | 3 (3.1) | |
| NYHA | - | ||
| I | - | 1 (1.0) | |
| II | - | 1 (1.0) | |
| III | - | 2 (2.0) | |
| IV | - | 26 (26.5) | |
| IIIA | - | 34 (34.7) | |
| IIIB | - | 34 (34.7) | |
| HF type | - | ||
| HFrEF | - | 97 (99.0) | |
| HFmrEF | - | 1 (1.0) | |
| INR | - | ||
| Basic INR | - | 1.21 ± 0.36 | |
| Target INR | - | 2.39 ± 0.26 | |
| Device strategy | - | ||
| BTT | - | 10 (10.2) | |
| DT | - | 88 (89.8) | |
| Device type | - | ||
| HW | - | 18 (18.4) | |
| HM2 | - | 34 (34.7) | |
| HM3 | - | 46 (46.9) | |
| Warfarin dose (mg/day) | - | 2.99 ± 1.15 | |
| Duration of LVAD support till outcome, from 2011 untill 2016, n = 36 (in months) | - | 29.6 ± 17.3 | |
| Patients’ achieved outcome till 2017 | - | ||
| Survived | - | 71 (72.4) | |
| Not-survived | - | 27 (27.6) | |
| Thrombosis | - | ||
| Yes | - | 13 (13.3) | |
| No | - | 85 (86.7) | |
| Bleeding | - | ||
| Yes | - | 14 (14.3) | |
| No | - | 84 (85.7) | |
| Infections | - | ||
| Yes | - | 39 (39.8) | |
| No | - | 59 (60.2) | |
| Stroke | - | ||
| No Stroke | - | 78 (79.6) | |
| Hemorrhagic stroke | - | 8 (8.2) | |
| Ischemic stroke | - | 12 (12.2) | |
| Myocardial infarction | - | ||
| Yes | - | 44 (44.9) | |
| No | - | 54 (55.1) | |
Continuous variables are presented, mean ± SD and categorical variables as n (%). HF patients, heart failure patients; Student’s t-test p value is labeled with one asterisk (*); Mann-Whitney U test’s p- value is labeled with double asterisks (**); The significant p value (p < 0.05) is labeled in bold; “-”, non-available parameters; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ICM, ischemic cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; VHD, valvular heart disease; NYHA, New York Heart Association; HFrEF, heart failure reduced ejection fraction; HFmrEF, heart failure mid-range ejection fraction; INR, International normalized ratio; BTT, bridge-to-transplantation; DT, destination therapy; HW, HeartWare HVAD; HM2, HeartMate II; HM3, HeartMate III.
After LVAD implantation, warfarin and aspirin were prescribed according to the clinical protocol of the Ministry of Healthcare of the Republic of Kazakhstan. Warfarin dose was corrected to maintain an international normalized ratio range (INR 2.25–3.25). Twenty-four (24.5%) patients suffered one of the post-implantation complications, such as thrombosis, bleeding and infections. Three patients had both thrombosis and bleeding complications.
Patients were categorized into two groups for comparative analysis: Group 1 (n = 74, without complications), and Group 2 (n = 24, with complications). Baseline demographic characteristics were compared between these two groups (Table 2); biochemical parameters also were compared between these two groups (Table S1).
Table 2.
Comparison of baseline demographic characteristics of patients with/without complications.
| Characteristic | Comparison between HF Patients | ||
|---|---|---|---|
| Group 1, N = 74 | Group 2, N = 24 | p Value | |
| Age (years) | 52.5 ± 11.3 | 53.4 ± 10.1 | 0.92 ** |
| Gender | |||
| Male | 71 (95.9) | 21 (87.5) | 0.16 |
| Female | 3 (4.1) | 3 (12.5) | |
| Ethnicity | |||
| Asian | 56 (75.7) | 21 (87.5) | 0.27 |
| Caucasian | 18 (24.3) | 3 (12.5) | |
| Body weight (kg) | 80.0 ± 12.2 | 79.3 ± 18.5 | 0.86 * |
| Height (cm) | 170.0 ± 6.08 | 168.9 ± 7.24 | 0.46 * |
| BMI (kg/m) | 27.7 ± 4.10 | 27.6 ± 5.66 | 0.97 * |
| SBP | 105.0 ± 15.7 | 104.0 ± 15.0 | 0.99 ** |
| DBP | 70.9 ± 10.7 | 72.1 ± 9.35 | 0.33 ** |
| History of smoking | |||
| Smokers | 46 (62.2) | 12 (50.0) | 0.34 |
| Non-smokers | 28 (37.8) | 12 (50.0) | |
| Diagnosis | |||
| ICM | 36 (48.6) | 8 (33.3) | 0.10 |
| DCM | 25 (33.8) | 15 (62.5) | |
| HCM | 10 (13.5) | 1 (4.2) | |
| VHD | 3 (4.1) | 0 | |
| NYHA | |||
| I | 1 (1.4) | 0 | 0.58 |
| II | 1 (1.4) | 0 | |
| III | 1 (1.4) | 1 (4.2) | |
| IV | 17 (23.0) | 9 (37.5) | |
| IIIA | 27 (36.5) | 7 (29.2) | |
| IIIB | 27 (36.5) | 7 (29.2) | |
| HF type | |||
| HFrEF | 74 (100) | 23 (95.8) | 0.25 |
| HFmrEF | 0 | 1 (4.2) | |
| INR | |||
| Basic INR | 1.19 ± 0.37 | 1.26 ± 0.33 | 0.11 ** |
| Target INR | 2.36 ± 0.24 | 2.46 ± 0.32 | 0.06 ** |
| Device strategy | |||
| BTT | 6 (8.1) | 4 (16.7) | 0.25 |
| DT | 68 (91.9) | 20 (83.3) | |
| Device type | |||
| HW | 11 (14.9) | 7 (29.2) | 0.01 |
| HM2 | 22 (29.7) | 12 (50.0) | |
| HM3 | 41 (55.4) | 5 (20.8) | |
| Warfarin dose (mg/day) | 3.01 ± 1.04 | 2.92 ± 1.46 | 0.29 ** |
| Duration of LVAD support till outcome, from 2011 untill 2016, n = 36 (in months) | 29.1 ± 17.6 (n = 21) | 30.3 ± 17.5 (n = 15) | 0.84 * |
| Patients’ achieved outcome till 2017 | |||
| Survived | 58 (78.4) | 13 (54.2) | 0.03 |
| Not-survived | 16 (21.6) | 11 (45.8) | |
| Thrombosis | |||
| Yes | 0 | 13 (54.2) | 0.0001 |
| No | 74 (100) | 11 (45.8) | |
| Bleeding | |||
| Yes | 0 | 14 (58.3) | 0.0001 |
| No | 74 (100) | 10 (41.7) | |
| Infections | |||
| Yes | 24 (32.4) | 15 (62.5) | 0.015 |
| No | 50 (67.6) | 9 (37.5) | |
| Stroke | |||
| No Stroke | 60 (81.1) | 18 (75.0) | 0.57 |
| Hemorrhagic stroke | 5 (6.8) | 3 (12.5) | |
| Ischemic stroke | 9 (12.2) | 3 (12.5) | |
| Myocardial infarction | |||
| Yes | 36 (48.6) | 8 (33.3) | 0.24 |
| No | 38 (51.4) | 16 (66.7) | |
Continuous variables are presented, mean ± SD and categorical variables as n (%). HF patients, heart failure patients; Group 1, without complications; Group 2, with complications; Student’s t-test p value is labeled with one asterisk (*); Mann-Whitney U test’s p- value is labeled is labeled with double asterisks (**); The significant p value (p < 0.05) is labeled in bold; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ICM, ischemic cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; VHD, valvular heart disease; NYHA, New York Heart Association; HFrEF, heart failure reduced ejection fraction; HFmrEF, heart failure mid-range ejection fraction; INR, International normalized ratio; BTT, bridge-to-transplantation; DT, destination therapy; HW, HeartWare HVAD; HM2, HeartMate II; HM3, HeartMate III.
For more detailed analysis, the group of patients with complications (Group 2) was classified into two subgroups: with thrombosis (Group 2-1) and bleeding (Group 2-2). Baseline demographic characteristics were analyzed comparing these two subgroup of patients with complications (Table S2). Comparison of biochemical parameters was further analyzed comparing Group 1 (without complications) and subgroup 2-1 (thrombosis), and subgroup 2-2 (bleeding), respectively (Table 3).
Table 3.
Comparative analysis of biochemical parameters between HF patients without complications, with thrombosis and bleeding complications.
| Study Groups | Parameters | Before 14 Days | p-Value | After 3–6 Months | p-Value | After 12–18 Month | p-Value |
|---|---|---|---|---|---|---|---|
| Group 1 | Hemoglobin, g/L | 139.6 ± 18.2 | 129.5 ± 16.2 | 125.7 ± 20.0 | |||
| Group 2-1 | 145.8 ± 21.7 | 0.32 * | 129.0 ± 15.3 | 0.93 * | 125.1 ± 18.4 | 0.95 * | |
| Group 2-2 | 128.5 ± 21.2 | 0.052 * | 111.1 ± 29.2 | 0.04 ** | 117.6 ± 35.0 | 0.55 * | |
| Group 1 | Hematocrit, % | 41.1 ± 6.72 | 38.2 ± 4.92 | 37.5 ± 5.46 | |||
| Group 2-1 | 43.1 ± 6.95 | 0.39 * | 37.0 ± 3.67 | 0.51 * | 36.3 ± 6.14 | 0.58 * | |
| Group 2-2 | 39.0 ± 6.61 | 0.30 * | 32.8 ± 8.70 | 0.016 * | 35.6 ± 10.3 | 0.63 * | |
| Group 1 | Lymphocytes, % | 27.5 ± 9.25 | 26.2 ± 8.79 | 22.0 ± 6.44 | |||
| Group 2-1 | 31.0 ± 8.78 | 0.19 ** | 18.4 ± 6.34 | 0.015 * | 29.8 ± 11.3 | 0.03 ** | |
| Group 2-2 | 21.8 ± 6.28 | 0.02 ** | 24.8 ± 9.35 | 0.68 * | 19.9 ± 11.3 | 0.27 ** | |
| Group 1 | LDH, U/L | 271.5 ± 137.0 | 273.5 ± 93.7 | 306.0 ± 156.1 | |||
| Group 2-1 | N/A | 335.4 ± 133.3 | 0.24 ** | 194.6 ± 42.7 | 0.02 ** | ||
| Group 2-2 | 254.0 ± 171.9 | 0.38 ** | 260.2 ± 139.0 | 0.78 * | 377.4 ± 174.2 | 0.19 ** | |
| Group 1 | AST, U/L | 27.9 ± 21.4 | 19.6 ± 5.50 | 21.2 ± 8.08 | |||
| Group 2-1 | 29.5 ± 10.6 | 0.11 ** | 26.9 ± 19.0 | 0.42 ** | 22.2 ± 8.44 | 0.77 * | |
| Group 2-2 | 33.3 ± 27.0 | 0.43 ** | 36.9 ± 37.5 | 0.02 ** | 43.0 ± 70.3 | 0.63 ** | |
| Group 1 | Total bilirubin, mg/dL | 1.33 ± 1.56 | 0.70 ± 0.51 | 1.03 ± 1.51 | |||
| Group 2-1 | 1.03 ± 0.73 | 0.74 ** | 1.26 ± 0.58 | 0.01 ** | 0.76 ± 0.80 | 0.55 ** | |
| Group 2-2 | 3.00 ± 5.09 | 0.40 ** | 1.59 ± 2.76 | 0.77 ** | 0.96 ± 0.71 | 0.42 ** |
Continuous variables are presented, mean ± SD. Student’s t-test p-value is labeled with one asterisk (*); Mann-Whitney U test’s p-value is labeled with double asterisks (**); the significant p value (p < 0.05) is labeled in bold; N/A, Not available. Group 1, without complications; Group 2-1, thrombosis; Group 2-2, bleeding; LDH, lactate dehydrogenase; AST, aspartate aminotransferase.
Ninety-five healthy individuals without any cardiovascular diseases at the time of recruitment (63 males and 32 females, 44.01 ± 13.8 years old) were included in the study as a control group for genetic analysis, representing the general population.
Clinical and epidemiological data were retrieved from medical records of patients at NRCC. Data included demographic parameters (age, gender, ethnicity), anthropometry (height, weight, body mass index (BMI)), LVAD type, systolic blood pressure (SBP), diastolic blood pressure (DBP), device type, echocardiography, thrombo-elastography (TEG), clinical biochemistry, including coagulation factors, lactase dehydrogenase (LDH), lupus anticoagulant (LA), and others.
Furthermore, we decided to compare patients without complications (Group 1) with thrombosis (Group 2-1) and bleeding (Group 2-2) subgroups, respectively (Table 3). Patients with bleeding events had significantly lower level of hemoglobin, hematocrit, lymphocytes and AST than Group 1 (p < 0.05). In addition, patients with thrombosis had significantly different values of lymphocytes, LDH and total bilirubin than those in Group 1 (p < 0.05). Other biochemical parameters did not show any statistically significant difference on the occurrence of complications (Table S4).
2.2. Selection of the SNPs
Twenty-one SNPs in VKORC1 (rs8050894, rs9934438, rs9923231), CYP2C9 (rs1799853, rs1057910, rs28371686), CYP2C19 (rs4244285, rs4986893), ITGB3 (rs5918), GGCX (rs11676382), CYP4F2 (rs2108622), UGT1A6 (rs2070959), ACSM2A (rs1133607), PTGS1 (rs3842787), F5 (rs6025), F13A1 (rs5985), F2 (rs1799963), F7 (rs6046), FGB (rs1800790), MTHFR (rs1801133, rs1801131) genes encoding coagulation system, metabolism of warfarin and aspirin, and previously showing association with cardiovascular events, were selected in this study [9,18,19,20]. We analysed known polymorphisms of VKORC1, CYP2C9, GGCX and CYP4F2 genes which are involved in warfarin metabolism and studied in HF patients with warfarin dosing in anticoagulant treatment [9,11,13,21,22,23]. Polymorphisms in genes CYP2C19, ITGB3, UGT1A6, ACSM2A, PTGS1 were included in the research based on their association with the antiplatelet effect of aspirin in cardiovascular events [17,18,24,25]. Genes of coagulation factors such as F5, F13A1, F2, F7, FGB and MTHFR involved in the folate/homocysteine metabolism pathway were selected due to their associations with thrombosis events [25,26,27]. A list of SNPs and used primers are summarized in Table S3.
2.3. DNA Extraction and SNP Genotyping
DNA extraction and genotyping were processed at the National Laboratory Astana, Nazarbayev University, Nur-Sultan, Kazakhstan. Genomic DNA was extracted from 200 μL whole blood samples using the PureLinkTM Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The concentration and purity of the extracted DNA were measured by NanoDrop™ Spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Further, DNA samples were genotyped by using real-time polymerase chain reaction (qPCR) with allele discrimination using TaqMan Real Time PCR Assay on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). qPCR mixture contained Universal Master Mix (2×) (Applied Biosystems, Waltham, MA, USA), 0.25 µL of TaqMan probe (40×) respectively (Applied Biosystems, Waltham, MA, USA) and 10 ng of DNA. Reaction volume was achieved with mqH2O. Further, qPCR results were determined by using SDS version 2.4 software.
2.4. Statistical Analysis
Continuous variables were presented as mean ± standard deviation (SD). Categorical variables were performed as percentages (%) and compared using chi-square test or Fisher’s exact test. Normality of distribution of continuous variables was assessed by using a Kolmogorov-Smirnov test (p > 0.05). Continuous variables were compared between two groups by Student’s t-test (normally distributed variables) and by non-parametric Mann—Whitney U test (not normally distributed variables). Sample size calculation and power analysis were identified by online calculator on https://clincalc.com, accessed on 5 september 2021. Sample sizes in the group of 75.5% (n = 74, without complications) and 24.5% (n = 24, with complications) achieved 0.85 (85%) power, with alpha value of 0.05. Each group needs at least 16 patients according to the sample size calculation results.
Hardy-Weinberg equilibrium (HWE) for genetic deviation was performed using chi square test or Fisher’s exact test. Associations between polymorphisms and HF patients with/without complications were evaluated by using odds ratios (OR) with 95% confidence interval (CI) and p value. Logistic regression analysis was performed using the web tool https://snpstats.net/, accessed on 9 March 2022. Bonferroni adjusted p-values were calculated for association of SNPs between multiple comparisons of healthy control group and HF patients with/without complications. A one-way ANOVA was done between genotype groups of statistically significant SNP genotypes. p-value < 0.05 was considered statistically significant. The statistical analysis was performed in SPSS program version 23 (SPSS, Chicago, IL, USA) Gene–gene interactions of all 21 SNPs in HF patients were investigated by Multifactor Dimensionality Reduction (MDR) analysis. MDR analysis was used for identification of low-risk and high-risk of genotypes. MDR analysis for 2 groups (with/without complications) of HF patients was assessed via R package. This package assumes binary case-control data with categorical predictor variables [28,29]. The binary response variable was coded as 0 or 1, and the categorical predictors (SNP genotypes) were coded numerically (0, 1, 2). The best MDR model was chosen by using a three-way split as internal validation methods to prevent over-fitting [29].
Furthermore, we studied the influence of genotype polymorphisms in the prediction of LVAD complications between HF patients (with/without complications) by using ROC analysis. ROC analysis was performed by using G-WIZ program in R [30]. To perform analysis by G-WIZ, we created file (csv.) with the study sample size, OR and risk allele frequencies for each SNP in the study.
3. Results
3.1. Clinical Characteristics of HF Patients with and without Complications
Baseline demographic characteristics of control group, HF patients and HF patients with and without complications are shown in Table 1 and Table 2. Among the 98 cases, 44 patients were diagnosed with ICM (44.9%) and 40 with DCM (40.8%) (Table 1). In our patient cohort, complications were more prevalent in patients with implanted HM2 device in 12 (50%) cases, and in patients with HW and HM3 in 7 cases (29.2) and 5 cases (20.8), respectively (p = 0.01) (Table 2). Most of the patients had implantation of LVAD as DT (89.8%), because heart donors were not available for every patient during the follow up period (Table 1). After implantation of the LVAD, 13 cases (54.2%) of thrombosis, 14 cases (58.3%) of bleeding and 15 (62.5%) of infection were developed in the group of patients with complications (p = 0.0001, p = 0.0001 and p = 0.015 for each type of complication respectively) (Table 2). The initial mean warfarin dose was 2.99 ± 1.15 mg/day and aspirin dose was prescribed a1s 00 mg daily.
In the period of this investigation (2011–2016), the mean duration of LVAD support was 29.6 ± 17.3 months. Seventy-one (72.4%) patients reached outcome measurement time, and among them, 10 (10.2%) patients had heart transplantation (BTT) (Table 1). Twenty-seven (27.6) patients died before reaching the time point of the outcome measurement (Table 1). In the group of patients who achieved outcome measurement, 58 (78.4%) had no complications (Group 1) and complications developed in 13 (54.2%) cases (Group 2), whereas among deceased patients complications developed in 11 cases (45.8%) (p = 0.03) (Table 2).
Biochemical parameters of pre- and post-LVAD implantation data were compared between patients with/without complications (Table S1). Hematocrit level after 6 months of LVAD implantation was significantly lower in the group of patients with complications than in those without complications (p = 0.047). According to the Mann-Whitney U test biochemical parameters such as lymphocytes, AST and total bilirubin level were significantly higher in the group of patients with complications (p < 0.05). The rest of the biochemical parameters did not show an effect on the development of complications (Table S1).
3.2. Analysis of Genotyping
Twenty-one SNPs were genotyped in HF patients (n = 98) with implanted LVAD and healthy controls (n = 95). The distributions of allelic and genotype frequencies among HF patients and controls, and among HF patients with/without complications, are summarized in Table 4 and Table 5. The distributions of allelic frequencies were tested for Hardy-Weinberg equilibrium (HWE).
Table 4.
The distributions of allelic and genotype frequencies of 21 SNPs between Control group and HF patients.
| Gene | SNP rs Number | Genotype | Control Group, No. (%) | Allele Frequency in Population Control Group | HF Patients, No. (%) | Allele Frequency in HF Patients | p Value |
|---|---|---|---|---|---|---|---|
| VKORC1*1 | rs8050894 | CC | 17 (17.9) | C:G = 0.39:0.61 | 55 (56.1) | C:G = 0.68:0.32 | 0.0001 * |
| CG | 41 (43.2) | 24 (24.5) | |||||
| GG | 37 (38.9) | 19 (19.4) | |||||
| C | 75 | 134 | |||||
| G | 115 | 62 | |||||
| ITGB3 | rs5918 | TT | 71 (74.7) | T:C = 0.87:0.13 | 48 (49.0) | T:C = 0.66:0.34 | 0.0001 * |
| TC | 23 (24.2) | 34 (34.7) | |||||
| CC | 1 (1.1) | 16 (16.3) | |||||
| T | 165 | 130 | |||||
| C | 25 | 66 |
Control group, healthy control group; HF patients, heart failure patients; p value of Hardy-Weinberg equilibrium test; p value (p < 0.05) numbers with statistical significance are labeled with asterisk (*) in bold.
Table 5.
The distributions of allelic and genotype frequencies of 21 SNPs between HF patients with/without complications.
| Gene | SNP rs Number | Genotype | Group 1, No. (%) | Allele Frequency in Group 1 | Group 2, No. (%) | Allele Frequency in Group 2 | p-Value |
|---|---|---|---|---|---|---|---|
| VKORC1*1 | rs8050894 | CC | 41 (55.4) | C:G = 0.68:0.32 | 14 (58.3) | C:G = 0.71:0.29 | 1 |
| CG | 18 (24.3) | 6 (25.0) | |||||
| GG | 15 (20.3) | 4 (16.7) | |||||
| C | 100 | 34 | |||||
| G | 48 | 14 | |||||
| VKORC1*2 | rs9934438 | GG | 14 (18.9) | G:A = 0.39:0.61 | 0 | G:A = 0.35:0.65 | 0.008 * |
| GA | 29 (39.2) | 17 (70.8) | |||||
| AA | 31 (41.9) | 7 (29.2) | |||||
| G | 57 | 17 | |||||
| A | 91 | 31 | |||||
| VKORC1*3 | rs9923231 | CC | 14 (18.9) | C:T = 0.40:0.60 | 0 | C:T = 0.35:0.65 | 0.012 * |
| CT | 31 (41.9) | 17 (70.8) | |||||
| TT | 29 (39.2) | 7 (29.2) | |||||
| C | 59 | 17 | |||||
| T | 89 | 31 | |||||
| CYP2C9*2 | rs1799853 | CC | 70 (94.6) | C:T = 0.97:0.03 | 21 (87.5) | C:T = 0.94:0.06 | 0.357 |
| CT | 4 (5.4) | 3 (12.5) | |||||
| TT | 0 | 0 | |||||
| C | 144 | 45 | |||||
| T | 4 | 3 | |||||
| CYP2C9*3 | rs1057910 | AA | 68 (91.9) | A:C = 0.96:0.04 | 23 (95.8) | A:C = 0.98:0.02 | 1 |
| AC | 6 (8.1) | 1 (4.2) | |||||
| CC | 0 | 0 | |||||
| A | 142 | 47 | |||||
| C | 6 | 1 | |||||
| CYP2C9*5 | rs28371686 | CC | 74 (100) | C:G = 1.000:0.000 | 24 (100) | C:G = 1.000:0.000 | N/A |
| CG | 0 | 0 | |||||
| GG | 0 | 0 | |||||
| C | 148 | 48 | |||||
| G | 0 | 0 | |||||
| CYP2C19*2 | rs4244285 | GG | 52 (70.3) | G:A = 0.84:0.16 | 19 (79.2) | G:A = 0.90:0.10 | 0.772 |
| GA | 20 (27.0) | 5 (20.8) | |||||
| AA | 2 (2.7) | 0 | |||||
| G | 124 | 43 | |||||
| A | 24 | 5 | |||||
| CYP2C19*3 | rs4986893 | GG | 70 (94.6) | G:A = 0.97:0.03 | 23 (95.8) | G:A = 0.98:0.02 | 1 |
| GA | 4 (5.4) | 1 (4.2) | |||||
| AA | 0 | 0 | |||||
| G | 144 | 47 | |||||
| A | 4 | 1 | |||||
| ITGB3 | rs5918 | TT | 42 (56.8) | T:C = 0.70:0.30 | 6 (25.0) | T:C = 0.56:0.44 | 0.005 * |
| TC | 19 (25.7) | 15 (62.5) | |||||
| CC | 13 (17.6) | 3 (12.5) | |||||
| T | 103 | 27 | |||||
| C | 45 | 21 | |||||
| GGCX | rs11676382 | CC | 70 (94.6) | C:G = 0.97:0.03 | 24 (100) | C:G = 1.000:0.000 | 0.677 |
| CG | 3 (4.0) | 0 | |||||
| GG | 1 (1.4) | 0 | |||||
| C | 143 | 48 | |||||
| G | 5 | 0 | |||||
| CYP4F2 | rs2108622 | CC | 47 (63.5) | C:T = 0.78:0.22 | 13 (54.2) | C:T = 0.71:0.29 | 0.501 |
| CT | 22 (29.7) | 8 (33.3) | |||||
| TT | 5 (6.8) | 3 (12.5) | |||||
| C | 116 | 34 | |||||
| T | 32 | 14 | |||||
| UGT1A6 | rs2070959 | AA | 28 (37.8) | A:G = 0.66:0.34 | 12 (50.0) | A:G = 0.65: 0.35 | 0.03 * |
| AG | 41 (55.4) | 7 (29.2) | |||||
| GG | 5 (6.8) | 5 (20.8) | |||||
| A | 97 | 31 | |||||
| G | 51 | 17 | |||||
| ACSM2A | rs1133607 | CC | 44 (59.5) | C:T = 0.78:0.22 | 15 (62.5) | C:T = 0.79:0.21 | 1 |
| CT | 27 (36.5) | 8 (33.3) | |||||
| TT | 3 (4.0) | 1 (4.2) | |||||
| C | 115 | 38 | |||||
| T | 33 | 10 | |||||
| PTGS1 | rs3842787 | CC | 72 (97.3) | C:T = 0.99:0.01 | 23 (95.8) | C:T = 0.98:0.02 | 1 |
| CT | 2 (2.7) | 1 (4.2) | |||||
| TT | 0 | 0 | |||||
| C | 146 | 47 | |||||
| T | 2 | 1 | |||||
| F5 | rs6025 | CC | 73 (98.6) | C:T = 0.99:0.01 | 24 (100) | C:T = 1.000:0.000 | 1 |
| CT | 1 (1.4) | 0 | |||||
| TT | 0 | 0 | |||||
| C | 147 | 48 | |||||
| T | 1 | 0 | |||||
| F13A1 | rs5985 | CC | 53 (71.6) | C:A = 0.84:0.16 | 19 (79.2) | C:A = 0.90:0.10 | 0.879 |
| CA | 19 (25.7) | 5 (20.8) | |||||
| AA | 2 (2.7) | 0% | |||||
| C | 125 | 43 | |||||
| A | 23 | 5 | |||||
| F2 | rs1799963 | GG | 74 (100) | G:A = 1.000:0.000 | 24 (100) | G:A = 1.000:0.000 | N/A |
| GA | 0 | 0 | |||||
| AA | 0 | 0 | |||||
| G | 148 | 48 | |||||
| A | 0 | 0 | |||||
| F7 | rs6046 | GG | 57 (77.0) | G:A = 0.87:0.13 | 18 (75.0) | G:A = 0.85:0.15 | 1 |
| GA | 15 (20.3) | 5 (20.8) | |||||
| AA | 2 (2.7) | 1 (4.2) | |||||
| G | 129 | 41 | |||||
| A | 19 | 7 | |||||
| FGB | rs1800790 | GG | 49 (66.2) | G:A = 0.82:0.18 | 16 (66.7) | G:A = 0.81:0.19 | 1 |
| GA | 23 (31.1) | 7 (29.2) | |||||
| AA | 2 (2.7) | 1 (4.2) | |||||
| G | 121 | 39 | |||||
| A | 27 | 9 | |||||
| MTHFR*1 | rs1801133 | GG | 37 (50.0) | G:A = 0.70:0.30 | 10 (41.7) | G:A = 0.65:0.35 | 0.742 |
| GA | 30 (40.5) | 11 (45.8) | |||||
| AA | 7 (9.5) | 3 (12.5) | |||||
| G | 104 | 31 | |||||
| A | 44 | 17 | |||||
| MTHFR*2 | rs1801131 | TT | 43 (58.1) | T:G = 0.76:0.24 | 11 (45.8) | T:G = 0.69:0.31 | 0.589 |
| TG | 27 (36.5) | 11 (45.8) | |||||
| GG | 4 (5.4) | 2 (8.3) | |||||
| T | 113 | 33 | |||||
| G | 35 | 15 |
Group 1, without complications; Group 2, with complications; p value of Hardy-Weinberg equilibrium test; p value (p < 0.05) numbers with statistical significance are labeled with asterisk (*) in bold.
The distributions of allelic and genotype frequencies of two SNPs rs8050894 in the VKORC1 and rs5918 in the ITGB3 genes were significantly different between HF patients and healthy control groups (p < 0.05) (Table 4). CC genotype of rs8050894 polymorphism in VKORC1 C > G gene was significantly higher in HF patients than in the control group (56.1% vs. 17.9%, p = 0.0001). CC genotype of rs5918 polymorphism in ITGB3 T > C gene was also significantly higher in HF patients than in control group (16.3% vs.1.1%, p = 0.0001). Nineteen SNPs were not significantly different between HF patients and healthy control groups (p > 0.05). Distribution of genotypes and alleles of studied polymorphisms in our study correspond to the population level according to 1000 Genomes, Ensemble, and NCBI databases. Allele frequency levels of 21 polymorphisms in HF patients were more common to Asian and East Asian populations. Results of allele frequency of HF patients, healthy control group and other different populations are summarized in Supplementary Table S5.
Furthermore, we found that the distributions of allelic and genotype frequencies of SNPs rs9934438, rs9923231 in the VKORC1 gene, rs5918 in the ITGB3 gene and rs2070959 in the UGT1A6 gene were significantly different between HF patient groups with and without complications (Table 5). Carriers of the GA genotype for polymorphism rs9934438 in VKORC1 G > A gene and CT genotype for rs9923231 were significantly higher in the group of patients with complications than in those patients without complications (70.8 vs. 39.2 and 70.8% vs. 41.9%, p < 0.05). TC genotype of rs5918 in ITGB3 T > C gene was significantly higher in the group of patients with complications than that in patients without complications (62.5% vs. 25.7%, p = 0.005). GG genotype of rs2070959 in UGT1A6 A > G gene was significantly higher in the group of patients with complications than in patients without complications (20.8% vs. 6.8%, p = 0.03).
Logistic regression analysis (adjusted for age, BMI and gender) showed that SNPs rs9934438; rs9923231 in VKORC1, rs5918 in ITGB3 and rs2070959 in UGT1A6 are significantly associated with patients with complications (Table 6). GA genotype of rs9934438 and CT genotype of rs9923231 in VKORC1 gene showed significant association with complications [OR (95% CI): 3.96 (1.42–11.02), p = 0.0057 and (OR (95% CI): 3.55 (1.28–9.86), p = 0.011]) TC genotype of rs5918 in ITGB3 gene and GG genotype of rs2070959 in the UGT1A6 gene were also significantly associated with the group of patients with complications [(R (95% CI): 5.37 (1.79–16.16), p = 0.0056 and OR (95% CI): 4.40 (1.06–18.20), p = 0.044]. Logistic regression analysis results for SNPs are summarized in Table S6.
Table 6.
Association analysis of SNP genotypes with complications’ development in HF groups (adjusted for age, BMI and gender).
| Gene, SNP | Model | Genotype | Group 1, No. (%) |
Group 2, No. (%) |
OR (95% CI) | p-Value | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| VKORC1 rs9934438 | Codominant | A/A | 31 (41.9) | 7 (29.2) | 1.00 | 0.0015 * | 106.1 | 121.6 |
| G/A | 29 (39.2) | 17 (70.8) | 2.69 (0.95–7.63) | |||||
| G/G | 14 (18.9) | 0 (0) | 0.00 (0.00–NA) | |||||
| Dominant | A/A | 31 (41.9) | 7 (29.2) | 1.00 | 0.27 | 115.9 | 128.8 | |
| G/A-G/G | 43 (58.1) | 17 (70.8) | 1.73 (0.64–4.74) | |||||
| Recessive | A/A-G/A | 60 (81.1) | 24 (100) | 1.00 | 0.0023 | 107.8 | 120.7 | |
| G/G | 14 (18.9) | 0 (0) | 0.00 (0.00–NA) | |||||
| Overdominant | A/A-G/G | 45 (60.8) | 7 (29.2) | 1.00 | 0.0057 * | 109.4 | 122.4 | |
| G/A | 29 (39.2) | 17 (70.8) | 3.96 (1.42–11.02) | |||||
| Log-additive | --- | --- | --- | 0.85 (0.43–1.70) | 0.65 | 116.9 | 129.8 | |
| VKORC1 rs9923231 | Codominant | T/T | 29 (39.2) | 7 (29.2) | 1.00 | 0.0024 | 107 | 122.5 |
| C/T | 31 (41.9) | 17 (70.8) | 2.36 (0.83–6.70) | |||||
| C/C | 14 (18.9) | 0 (0) | 0.00 (0.00–NA) | |||||
| Dominant | T/T | 29 (39.2) | 7 (29.2) | 1.00 | 0.38 | 116.3 | 129.2 | |
| C/T-C/C | 45 (60.8) | 17 (70.8) | 1.56 (0.57–4.27) | |||||
| Recessive | T/T-C/T | 60 (81.1) | 24 (100) | 1.00 | 0.0023 | 107.8 | 120.7 | |
| C/C | 14 (18.9) | 0 (0) | 0.00 (0.00–NA) | |||||
| Overdominant | T/T-C/C | 43 (58.1) | 7 (29.2) | 1.00 | 0.011 * | 110.6 | 123.5 | |
| C/T | 31 (41.9) | 17 (70.8) | 3.55 (1.28–9.86) | |||||
| Log-additive | --- | --- | --- | 0.80 (0.40–1.62) | 0.54 | 116.7 | 129.6 | |
|
ITGB3
rs5918 |
Codominant | T/T | 42 (56.8) | 6 (25.0) | 1.00 | 0.0056 * | 108.7 | 124.2 |
| T/C | 19 (25.7) | 15 (62.5) | 5.37 (1.79–16.16) | |||||
| C/C | 13 (17.6) | 3 (12.5) | 1.47 (0.31–7.05) | |||||
| Dominant | T/T | 42 (56.8) | 6 (25.0) | 1.00 | 0.0079 * | 110 | 123 | |
| T/C-C/C | 32 (43.2) | 18 (75.0) | 3.83 (1.35–10.89) | |||||
| Recessive | T/T-T/C | 61 (82.4) | 21 (87.5) | 1.00 | 0.49 | 116.6 | 129.5 | |
| C/C | 13 (17.6) | 3 (12.5) | 0.62 (0.15–2.53) | |||||
| Overdominant | T/T-C/C | 55 (74.3) | 9 (37.5) | 1.00 | 0.0014 * | 106.9 | 119.9 | |
| T/C | 19 (25.7) | 15 (62.5) | 4.83 (1.79–13.06) | |||||
| Log-additive | --- | --- | --- | 1.59 (0.84–2.99) | 0.15 | 115 | 128 | |
| UGT1A6 rs2070959 | Codominant | A/A | 28 (37.8) | 12 (50) | 1.00 | 0.03 | 112.1 | 127.6 |
| A/G | 41 (55.4) | 7 (29.2) | 0.40 (0.13–1.17) | |||||
| G/G | 5 (6.8) | 5 (20.8) | 2.67 (0.59–12.07) | |||||
| Dominant | A/A | 28 (37.8) | 12 (50) | 1.00 | 0.29 | 116 | 128.9 | |
| A/G-G/G | 46 (62.2) | 12 (50) | 0.60 (0.23–1.56) | |||||
| Recessive | A/A-A/G | 69 (93.2) | 19 (79.2) | 1.00 | 0.044 * | 113 | 125.9 | |
| G/G | 5 (6.8) | 5 (20.8) | 4.40 (1.06–18.20) | |||||
| Overdominant | A/A-G/G | 33 (44.6) | 17 (70.8) | 1.00 | 0.02 * | 111.7 | 124.6 | |
| A/G | 41 (55.4) | 7 (29.2) | 0.32 (0.11–0.87) | |||||
| Log-additive | --- | --- | --- | 1.08 (0.51–2.29) | 0.84 | 117 | 130 |
Group 1, without complications; Group 2, with complications; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; The p value (p < 0.05) numbers with statistical significance are labeled with asterisk (*) in bold.
Polymorphisms of rs8050894 in VKORC1 and rs5918 in ITGB3 genes were found to be significantly associated with HF and complications according to the Bonferroni correction analysis (p < 0.001).
We performed MDR analysis to identify gene–gene interactions of 21 SNPs in the HF patients. We considered all combinations of 21 SNPs up to size K = 3 and default settings for the other options. The best MDR model combinations according to the three-way split internal validation are summarized in Table 7.
Table 7.
Summary table for MDR fit with three-way split validation (k = 3).
| Level | Best Models | SNP rs Number | Training Accuracy | Classification Accuracy | Prediction Accuracy |
|---|---|---|---|---|---|
| 1 | MTHFR*1 | rs1801133 | 69.70 | 68.23 | 50.00 |
| 2 |
VKORC1*2,
MTHFR*2 |
rs9934438, rs1801131 | 73.79 | 76.04 | 54.17 |
| *3 |
VKORC1*2,
UGT1A6, MTHFR*1 |
rs9934438, rs2070959, rs1801133 |
88.33 | 82.29 | 95.83 |
‘*’ indicates overall best model.
MDR fit identified that combinations of polymorphisms rs9934438 in VKORC1*2, rs2070959 in UGT1A6 and 1801133 in MTHFR*1 genes are the best model of disease prediction status (in our case, whether HF patients with/without complications) with prediction accuracy of 95.83% (Table 7).
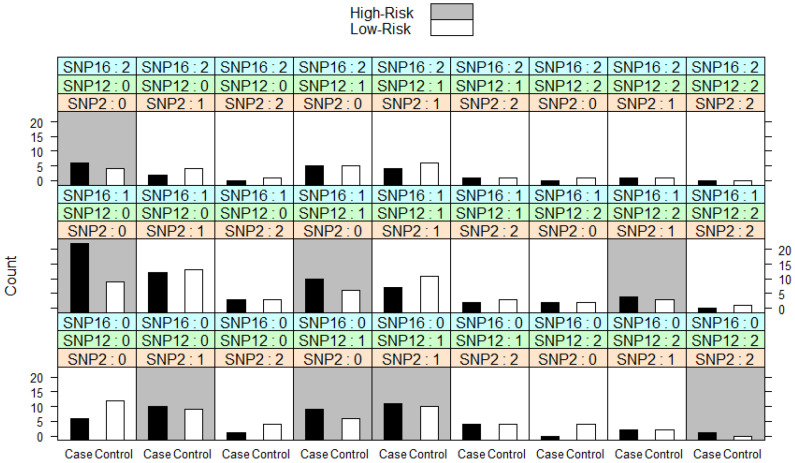
We can visually display the results for MDR fit and see which genotype combinations are at high-risk. In our case, eight combinations of polymorphisms rs9934438 in VKORC1*2 gene, rs2070959 in UGT1A6 gene and rs1801133 in MTHFR*1 gene are classified as high-risk for complications in HF patients (Figure 1).
Figure 1.
The illustration of MDR fit with three-way split on LVAD dataset with 98 individuals at 21 SNPs (k = 3). SNP 2 is polymorphism rs9934438 in VKORC1*2 gene; SNP12 is polymorphism rs2070959 in UGT1A6 gene; SNP16 is polymorphism rs1801133 in MTHFR*1.
Further, we repeated analysis with combination size for K = 4 (Table 8). This includes combinations of polymorphisms rs5918 in ITGB3 gene, rs2108622 in CYP4F2 gene, rs1801133 in MTHFR*1 gene and rs1800790 in FGB gene, which provides gave the best predictive status (96.77%) as to whether patients will experience complications or not. The plot of MDR fit was also extracted, but due to the large number of combinations the plot is presented in a supplementary file (Supplementary Figure S1).
Table 8.
Summary table for MDR fit with three-way split validation (k = 4).
| Level | Best Models | SNP rs Number | Training Accuracy | Classification Accuracy | Prediction Accuracy |
|---|---|---|---|---|---|
| 1 | ITGB3 | rs5918 | 66.82 | 83.18 | 83.18 |
| 2 |
VKORC1*2
ITGB3 |
rs9934438 rs5918 |
74.24 | 86.41 | 86.41 |
| 3 |
ITGB3
MTHFR*1 FGB |
rs5918 rs1801133 rs1800790 |
83.79 | 95.16 | 95.16 |
| *4 |
ITGB3
CYP4F2 MTHFR*1 FGB |
rs5918 rs2108622 rs1801133 rs1800790 |
92.12 | 96.77 | 96.77 |
‘*’ indicates overall best model.
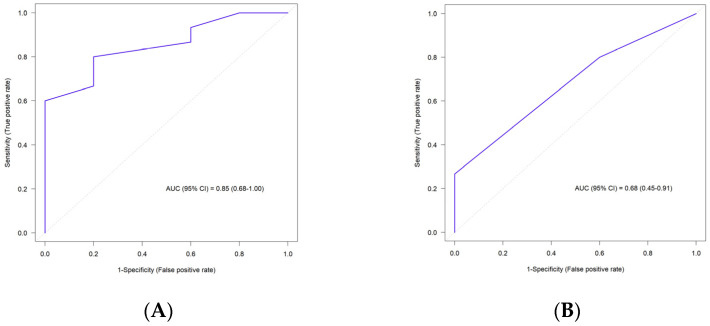
ROC analysis included the study sample size, OR, risk allele frequencies and model type for each SNP of the study (Table S7). ROC analysis was performed for 19 SNPs except polymorphisms of rs28371686 in CYP2C9*5 gene and rs1799963 in F2 gene. HF patients were carriers only of wild genotypes for rs28371686 in CYP2C9*5 and rs1799963 in F2 genes. The ROC curve for 19 SNPs is plotted in Figure 2A and the area under the ROC curve was 0.85 which showed a good discriminator between HF patients with and without complications. Based on this ROC curve and AUC score, we could claim that 19 SNPs have good predictive value on HF patients’ complications status.
Figure 2.
ROC plots for a model containing SNP variants for HF patients. (A) ROC plot for a model containing 19 SNP variants for HF patients (AUC = 0.85). (B) ROC plot for a model containing 4 SNP variants for HF patients (AUC = 0.68).
We also performed ROC analysis for four SNPs rs9934438, rs9923231 in VKORC1 gene, rs5918 in ITGB3 gene and rs2070959 in UGT1A6 gene, which were significantly associated with HF patients’ complications according to the logistic regression analysis (Table 6). The ROC curve is demonstrated in Figure 2B. AUC score for 4 SNPs was 0.68 which was lower in comparison to the ROC curve for 19 SNPs.
Significantly different SNPs rs8050894, rs9934438, rs9923231 in VKORC1, rs5918 in ITGB3 and rs2070959 in UGT1A6 were analyzed in HF patients with and without complications. We performed analyses of biochemical parameter levels according to genotypes of SNPs rs8050894, rs9934438, rs9923231 in VKORC1, rs5918 in ITGB3 and rs2070959 in UGT1A6 respectively in patients with- and without- complications (Table S8). Biochemical parameters such as hemoglobin, hematocrit, leukocytes, lymphocytes, erythrocytes, prothrombin time, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, blood urea nitrogen (BUN) and other characteristics were significantly associated with three SNPs (rs8050894, rs9934438, rs9923231) in VKORC1, one SNP (rs5918) in ITGB3 and one SNP (rs2070959) in UGT1A6 genes (p < 0.05).
4. Discussion
This study aimed to investigate the genotype distributions of 21 SNPs which are involved in the coagulation system, warfarin and aspirin metabolism in HF patients with/without complications due to LVAD implantation, and in a healthy control group. We selected SNPs which were studied previously in HF patients and in other cardiovascular events. Polymorphisms of VKORC1 and CYP2C9 genes were studied previously in LVAD patients [9,11]. However, we did not find in the available literature that other SNPs were investigated specifically in HF patients with implanted LVAD [23,25]. Distribution of genotypes and alleles of all 21 SNPs in our study correspond to the population level according to 1000 Genomes, Ensemble, and NCBI databases, which showed a similar frequency to Asian and East Asian populations (Supplementary Table S5).
Out of 21 SNPs, distributions of the allelic and genotypic frequencies of polymorphisms rs8050894 in VKORC1 gene and rs5918 in ITGB3 gene were significantly different between HF patients and healthy controls (Bonferroni correction analysis (p < 0.001)). In addition, we found significant differences in distributions of the allelic and genotypic frequencies of polymorphisms rs9934438, and rs9923231 in VKORC1 gene, rs5918 in ITGB3 gene and rs2070959 in UGT1A6 gene between HF patients with and without complications. Logistic regression analysis revealed significant association of rs9934438; rs9923231 in VKORC1, rs5918 in ITGB3 and rs2070959 in UGT1A6 with complications of HF patients. These SNPs were further analyzed in detail for identification their genetic influence on HF patients with/without- complications.
We performed MDR analysis to identify gene–gene interactions of 21 SNPs in the HF patients. MDR fit identified that combinations of polymorphisms rs9934438 in VKORC1*2, rs2070959 in UGT1A6 and 1801133 in MTHFR*1 genes are the best model of disease prediction status (in our case, whether HF patients were with/without complications) with prediction accuracy of 95.83% (Table 7).
Warfarin’s metabolism and dose effects depend on genetic variants of vitamin K epoxide reductase complex subunit 1 (VKORC1) [1,9,11,19]. Genotypic polymorphisms of VKORC1 gene could help to prevent over- and under-coagulation by predicting what warfarin dosage is optimal, which can reduce thrombosis and bleeding complications [9,11].
VKORC1 polymorphisms have different genotypic distributions across different racial groups such as African-American, European-American, Asian and Caucasian [9,11,20,21]. Scott et al. identified significantly different allelic frequency of polymorphism rs9923231 in VKORC1 gene between African-American and Asian populations (p < 0.0001). Investigation revealed that an Asian population had significantly higher frequency of mutant allele (-1639A) of polymorphism rs9923231 in VKORC1 gene than wild type allele (-1639G) [20]. Our study also showed that healthy control group and HF patients have higher frequency of mutant T allele of polymorphism rs9923231 in VKORC1 gene similar to Asian population (Table S5). Moreover heterozygote genotype polymorphisms for rs9934438 and rs9923231 in VKORC1gene are significantly associated with the group of HF patients with complications (p < 0.05).
A higher warfarin dose is recommended with the presence of the wild type genotypic polymorphisms in VKORC1 gene [9,11]. On the other hand, lower warfarin dose is usually prescribed with the presence of the mutant genotype polymorphisms [9,19,22,31]. Our research found significant difference of prescribed warfarin dosage in the clinic between genotype polymorphisms of rs8050894, rs9934438, rs9923231 in VKORC1 gene among HF patients with LVAD implantation (p < 0.05) (Table S9). HF patients with the presence of the wild type GG genotype and heterozygote GA genotype for rs9934438 in VKORC1 gene were prescribed with higher warfarin dosage with maximum of 3.88 ± 1.25 mg/day. HF patients; carriers of mutant AA genotype for rs9934438 were prescribed with lower warfarin dose with maximum of 2.44 ± 0.81 mg/day. However, in our research we did not find any significant association of genotype polymorphisms in VKORC1 gene separately with thrombosis and bleeding complication groups (p < 0.05). On the contrary, Topkara et al. (2015) found that HF patients had significantly higher risk of thrombosis complications with mutant genotype polymorphism of rs9923231 in VKORC1 gene than with wild type genotype. However, there was no association with gastrointestinal bleeding complications in HF patients with implanted LVAD [32]. Warfarin dose difference for each genotype polymorphisms rs8050894, rs9934438, rs9923231 in VKORC1 gene for HF patients is summarized in Table S9. Our result supported that genotype guided warfarin dosage before prescription will reduce both mortality and development of complications in HF patients (thrombosis, bleeding) after LVAD implantation, consistent with previous reports [9,11].
LVAD’s shear stress causes platelet dysfunction which affects normal hemostatic function. Investigations revealed that high non-physiologic shear stress (NPSS) causes loss of the GPIbα platelet glycoprotein receptor which affects increased bleeding events in HF patients with implanted LVAD [33]. NPSS also causes thrombosis complications by enhanced activation of GPIIb/IIIa receptors [14,34]. If NPSS causes damage of the platelet receptors, the genetic polymorphism of the receptors also needs to be considered [35]. Platelet’s function may vary by 30% due to genetic diversity. Genetic polymorphisms of platelet receptors have associations with high risk of bleeding and thrombosis events [17,35,36]. Genetic factors of glycoprotein GPIIb/IIIa receptors, fibrinogen, prothrombin, VWF, factor V, factor VII, factor XIII and plasminogen activator inhibitor-1 have major role in the process of hemostasis and are associated with side effects in cardiovascular events. Consequently, these genetic factors influence the treatment outcome in cardiovascular diseases [16,17,18,35,37,38].
This current investigation also included the polymorphism rs5918 of ITGB3 gene which encodes platelet receptor GPIIIa [17,38]. There are reports that mutant CC genotype of rs5918 in the ITGB3 gene has higher risk of thrombosis formation in cases of stroke, coronary artery disease and myocardial infarction (MI) [18,38,39,40,41]. Our study showed that only heterozygote TC genotype of rs5918 in ITGB3 gene was significantly associated with HF patients who have LVAD complications such as thrombosis and bleeding (n = 24) (OR (95% CI): 5.37 (1.79–16.16), p = 0.0056). Apart from the side effects of NPSS, platelet receptor GPIIIa with TC genotype of rs5918 in ITGB3 gene could have heritable platelet dysfunction which causes higher risk of complications in HF patients with implanted LVAD. Previous investigations did not consider genes encoding platelet receptors for the predictions of the complications in HF patients with implanted LVAD. Identification of the warfarin dose by polymorphisms of VKORC1 and CYP2C9 genes is not enough for the prediction and prevention of complications [9,11]
Acetyl salicylic acid (aspirin) is one of the most effective nonsteroidal anti-inflammatory drugs (NSAID), which deacetylates to salicylic acid after absorption. The metabolism of aspirin occurs in two phases: (1) Phase-I by enzyme of cytochrome P450s; (2) Phase-II by glucuronidation which is catalyzed by UDP-glucuronosyltransferases (UGTs) [24,42,43]. UGT1A6 is a major enzyme involved in aspirin metabolism. Genetic polymorphism in UGT1A6 can influence to the expression of different metabolic activities of the enzyme [24,43,44]. UGT1A6 gene polymorphism rs2070959 was found to be associated with colon and colorectal cancer [24,45,46,47]. Furthermore, UGT1A6 gene polymorphism rs2070959 was found not to be associated in cardiovascular patients with gastrointestinal complaints in aspirin treatment [42].
Aspirin metabolites excrete faster in individuals with GG genotype of UGT1A6 rs2070959, and on the contrary, UGT1A6 rs2070959 AA genotype carriers excrete a smaller amount of aspirin metabolites because of slower metabolism [44,45]. Patients with GG genotype require a higher aspirin dose due to the enzyme’s higher metabolic activity [44]. However, there were controversial findings regarding this SNP, showing that GG genotype polymorphism rs2070959 in UGT1A6 gene is associated with decreased enzyme activity [46,48].
In our study, distribution of mutant GG genotype of rs2070959 in the UGT1A was significantly associated with HF patients with complications (OR (95% CI): 2.67 (0.59–12.07), p = 0.03). Distribution of AA genotype of rs2070959 in the UGT1A6 gene was higher in HF patients with complications than those patients without complications (50% vs. 37.8%). Patients (n = 24) had development of complications such as pump thrombosis, stroke, and internal jugular vein thrombosis, on average after 24.5 months of LVAD implantation (from 7 months to 54 months). There were also complications in the form of bleeding from the gastrointestinal tract, gynecological, nasal, rectal bleeding and gingival bleeding, which developed on average 9.4 months after implantation (from 1 month to 24 months). These complications in several patients were even diagnosed twice after the implantation of the device.
According to the HF patients’ listed complications and their genotypes, our research found that 100mg of aspirin was not enough for patients with GG genotype of rs2070959 in the UGT1A6 gene, which excretes faster aspirin metabolites. On the contrast, the same amount of aspirin was high for patients with AA genotype of rs2070959 in the UGT1A6 gene, which is responsible for the slower excretion of aspirin metabolites. Identification of genotypes of rs2070959 in UGT1A6 gene could help to guide the prescription of aspirin individual dosage for reduction of complications before and after LVAD implantation. Various UGT polymorphisms should be studied for the identification of patient’s precise aspirin dosage, which will help to reduce complications and mortality rate [43]. Polymorphism rs2070959 in UGT1A6 gene is the first polymorphism studied in HF patients with thrombosis and bleeding complications after implanted LVAD devices [42,45,47,49]. LVAD patients should be prescribed with individualized aspirin dosage according to the results of genotyping for the polymorphisms of UGT1A6 gene.
We also analyzed biochemical parameters between control, thrombosis and bleeding subgroups (Supplementary Table S4). Patients with bleeding events had significant differences in hemoglobin, hematocrit and lymphocyte levels compared to patients without complications (p < 0.05). Significant reduction of hemoglobin and hematocrit levels were identified in patients with bleeding events [7,50]. In this study, we showed that HF patients with bleeding complications had significantly lower level of hemoglobin (111.1 ± 29.2 vs. 129.5 ± 16.2, p = 0.012) and lower level of hematocrit (32.8 ± 8.70 vs. 38.2 ± 4.92, p = 0.016) than those HF patients without complications at post-LVAD (p < 0.05). On the other hand, HF patients with thrombosis complications had significantly different levels of lymphocytes and total bilirubin (p < 0.05). There are investigations showing that a lower level of hematocrit could be a predictor of bleeding events pre-LVAD [50]. Our results showed that lower levels of hemoglobin and hematocrit could be a predictor of bleeding complications at both pre- and post-LVAD implantation period. Biochemical parameters can predict development of complications before LVAD implantation and such biochemical measurements might help to reduce mortality rate among HF patients.
Furthermore, we compared biochemical parameters between carriers of different genotypes of polymorphisms rs8050894, rs9934438, rs9923231 in VKORC1 gene, rs5918 in ITGB3 gene and rs2070959 in UGT1A6 gene for identification of genetic influence among HF patients with/without complications (Supplementary Table S8). Level of fibrinogen, hemoglobin, hematocrit, blood urea nitrogen, leukocytes, lymphocytes, total bilirubin and other biochemical parameters were significantly associated with the genotypes of VKORC1, ITGB3 and UGT1A6 genes (p < 0.05). Previously, we mentioned that lower level of hemoglobin (111.1 ± 29.2, p = 0.012) and hematocrit (32.8 ± 8.70, p = 0.016) could be a predictor of the bleeding complications in HF patients after LVAD implantation (Table 3). On the other hand, levels of hemoglobin (103.7 ± 9.61, p = 0.01) and hematocrit (32.3 ± 4.73, p = 0.01) were significantly lower in GG genotype polymorphisms of rs8050894 in VKORC1 gene (p < 0.05). Our study identified that patients with GG genotype of rs8050894 in VKORC1 gene could have lower level of hematocrit and hemoglobin with risks of bleeding complications after LVAD implantation.
Prothrombin time was also significantly different in carriers of genotypes CC and TT rs5918 of ITGB3 gene (38.4 ± 20.4 vs. 23.9 ± 5.96, p = 0.03, respectively). Platelet receptor subunits GPIIb/IIIa are encoded with ITGA2B (GPIIb) and ITGB3 (GPIIIa) genes. ITGB3 and ITGA2B gene polymorphisms are associated with increased platelet activity. Defect of the platelet receptors due to genetic inheritance can cause Glanzmann’s thrombasthenia, which is followed by disorders of platelet aggregation and increased bleeding events. Investigations showed that increased prothrombin time was shown to be associated with polymorphism rs70940817 of ITGB3 gene [51]. In future perspectives, our investigation should include other polymorphisms of ITGB3 gene and discover stronger associations with platelet dysfunctions in HF patients after LVAD implantation. Identification of different gene polymorphisms and biomarkers in LVAD patients could prevent LVAD complications and mortality and develop new pharmacological treatments for a better life for HF patients [52].
We observed that carriers of mutant GG genotype of rs2070959 in UGT1A6 gene had significantly higher level of bilirubin than wild type AA carriers (2.70 ± 2.35 vs. 0.88 ± 0.57, respectively, p = 0.04). Oussalah et al. (2015) in their research showed that polymorphisms of UGT1A6 gene were associated with serum bilirubin concentration [53]. Moreover, other studies demonstrate that UGT1A1*28 polymorphism is associated with hyperbilirubinemia in newborns [54]. UGT1A1*28 polymorphism was found to be the main cause of Gilbert syndrome which is identified with chronic hyperbilirubinemia [54,55]. Our findings showed that HF patients, carriers of mutant GG genotype, had a high bilirubin level, which will need more detailed analysis in future studies.. Other polymorphisms of the gene should be studied in HF patients with implanted LVAD to clarify the role of this gene and association with bilirubin level.
5. Limitations
Our study involved 98 HF patients with implanted LVADs. The small sample size limited the statistical analysis power in sub-groups analysis. The low sample size is problematic, especially when trying to create comprehensively adjusted models. Some of the biochemical parameters data were not longitudinally available in this current study due to patients’ death.
6. Conclusions
Polymorphisms of rs8050894, rs9934438, rs9923231 in VKORC1 gene, rs5918 in ITGB3 gene and rs2070959 in UGT1A6 gene showed significant association in HF patients who have LVAD with thrombosis and bleeding complications. Genotyping of polymorphisms could help to predict complications at both pre- and post-LVAD implantation periods which will reduce morbidity and mortality rate. Our research showed that patients can receive personalized treatment with warfarin and/or aspirin dosage by reference to their genotypes in polymorphisms of VKORC1 and UGT1A6 genes.
We also found that HF patients with bleeding and thrombosis events have a significant difference in biochemical parameters at pre- and post-LVAD implantation period. Changes in biochemical parameters could predict complications after LVAD implantation. Biochemical parameters were significantly associated with genotypes of polymorphisms in VKORC1, ITGB3 and UGT1A6 genes.
This study for the first time investigated genetic polymorphisms of rs2070959 in UGT1A6 gene in HF patients with implanted LVAD devices in Kazakhstan. We concluded that LVAD complications can be predicted by genotyping of polymorphisms of genes which are involved in the coagulation system, hemostasis function and in the metabolism of the antithrombotic/anticoagulant therapy. Recent development of genomic technologies and decreasing high throughput sequencing price per Gb allows more wide applications of sequencing technologies in clinical practice. Next-generation sequencing technologies such as whole exome and whole genome sequencing could be useful in diagnosis of HF patients with implanted LVAD, prediction of complication risks and prevention. Machine learning models in addition to traditional logistic regression and other forms of analysis could achieve better capabilities of detecting risk genetic risk factors contributing to the development of heart failure and complications of antithrombotic and anticoagulant therapies [56]. Using NGS technologies and machine learning models in future perspective would prevent patients experiencing a high risk of complications, reduce mortality rate and shorten treatment days in-hospital stay after LVAD implantation. Genomic personalized treatment guided by innovative technologies will bring great achievements in treatment of HF patients before prescription of LVAD implantation.
Acknowledgments
We are grateful to Ulykbek Kairov and Aigul Sharip for their valuable help and assistance in the statistical analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12050744/s1, Table S1: Comparative analysis of biochemical parameters between HF patients with/without complications; Table S2: Baseline demographic characteristics between HF patients with thrombosis and bleeding complications; Table S3: List of SNP characteristics; Table S4: Comparative analysis of biochemical parameters between HF patients without complications, with thrombosis and bleeding complications; Table S5: The distributions of allelic and genotype frequencies of 21 SNPs between Control group and HF patients.; Association analysis of SNP genotypes with complications development in HF groups (adjusted for age, BMI and gender); Table S6: Association analysis of SNP genotypes with complications development in HF groups (adjusted for age, BMI and gender); Table S7: Summary of 19 SNP variants in 98 HF patients with-/without complications for ROC analysis; Table S8: Comparative analysis of biochemical parameters between genotypes; Table S9: Warfarin dosage between genotype polymorphisms of VKORC1 gene; Figure S1: The illustration of MDR fit with three-way split on LVAD dataset with 98 individuals at 21 SNPs, where k = 4. SNP 10 is polymorphism rs5918 in ITGB3 gene; SNP 11 is polymorphism rs2108622 in CYP4F2 gene; SNP12 is polymorphism rs1801133 in MTHFR*1 gene; SNP21 is polymorphism rs1800790 in FGB gene.
Author Contributions
Conceptualization, M.S.B. and A.R.A.; Data curation, M.R.Z., G.A.A., G.K.K., Y.V.P., M.S.B. and A.R.A.; Formal analysis, M.R.Z., G.A.A., K.R.A., J.H.L. and A.R.A.; Funding acquisition, A.R.A.; Investigation, M.R.Z., S.E.R., U.A.K., G.A.A. and G.K.K.; Methodology, M.R.Z., S.E.R., U.A.K., G.A.A., G.K.K., K.R.A., Y.V.P. and A.R.A.; Project administration, A.R.A.; Resources, M.S.B. and A.R.A.; Software, U.A.K., K.R.A. and J.H.L.; Supervision, A.R.A.; Validation, S.E.R., U.A.K., J.H.L. and A.R.A.; Writing—original draft, M.R.Z. and A.R.A.; Writing—review & editing, Y.V.P., J.H.L., M.S.B. and A.R.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of National Laboratory Astana, Nazarbayev University (protocol No.16 from 11 March 2015) and by the Ethics Committee of National Research Cardiac Surgery Center (NRCC), Nur-Sultan, Kazakhstan (protocol No.16 from 24 April 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. During the data collection, all personal information of patients is encoded and the data is depersonalized, so we protect the rights of patients and do not disclose their personal information. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
National Laboratory Astana (contact via phone or mail) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from the authors, phone number: +77172706501, mail: akilzhanova@nu.edu.kz.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by Ministry of Education and Science of the Republic of Kazakhstan, grant number AP09563474.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nakagita K., Wada K., Mukai Y., Uno T., Nishino R., Matsuda S., Takenaka H., Terakawa N., Oita A., Takada M. Effects of vitamin K epoxide reductase complex 1 gene polymorphisms on warfarin control in Japanese patients with left ventricular assist devices (LVAD) Eur. J. Clin. Pharmacol. 2018;74:885–894. doi: 10.1007/s00228-018-2483-8. [DOI] [PubMed] [Google Scholar]
- 2.Kadakia S., Moore R., Ambur V., Toyoda Y. Current status of the implantable LVAD. Gen. Thorac. Cardiovasc. Surg. 2016;64:501–508. doi: 10.1007/s11748-016-0671-y. [DOI] [PubMed] [Google Scholar]
- 3.Nascimbene A., Neelamegham S., Frazier O.H., Moake J.L., Dong J.F. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127:3133–3141. doi: 10.1182/blood-2015-10-636480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng A., Williamitis C.A., Slaughter M.S. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: Is there an advantage to pulsatility? Ann. Cardiothorac. Surg. 2014;3:573–581. doi: 10.3978/j.issn.2225-319X.2014.08.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pya Y., Bekbossynova M., Jetybayeva S., Bekbossynov S., Andossova S., Salov R., Medressova A., Novikova S., Murzagaliyev M. Initial 3-year outcomes with left ventricular assist devices in a country with a nascent heart transplantation program. ESC Heart Fail. 2016;3:26–34. doi: 10.1002/ehf2.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimpfer D., Netuka I., Schmitto J.D., Pya Y., Garbade J., Morshuis M., Beyersdorf F., Marasco S., Rao V., Damme L., et al. Multicentre clinical trial experience with the HeartMate 3 left ventricular assist device: 30-day outcomes. Eur. J. Cardiothorac. Surg. 2016;50:548–554. doi: 10.1093/ejcts/ezw169. [DOI] [PubMed] [Google Scholar]
- 7.Mondal N.K., Sorensen E.N., Hiivala N.J., Feller E.D., Pham S.M., Griffith B.P., Wu Z.J. Intraplatelet reactive oxygen species, mitochondrial damage and platelet apoptosis augment non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist device. Platelets. 2015;26:536–544. doi: 10.3109/09537104.2014.948840. [DOI] [PubMed] [Google Scholar]
- 8.Susen S., Rauch A., van Belle E., Vincentelli A., Lenting P.J. Circulatory support devices: Fundamental aspects and clinical management of bleeding and thrombosis. J. Thromb. Haemost. 2015;13:1757–1767. doi: 10.1111/jth.13120. [DOI] [PubMed] [Google Scholar]
- 9.Topkara V.K., Knotts R.J., Jennings D.L., Garan A.R., Levin A.P., Breskin A., Castagna F., Cagliostro B., Yuzefpolskaya M., Takeda K., et al. Effect of CYP2C9 and VKORC1 Gene Variants on Warfarin Response in Patients with Continuous-Flow Left Ventricular Assist Devices. ASAIO J. 2016;62:558–564. doi: 10.1097/MAT.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 10.Zimpfer D., Gustafsson F., Potapov E., Pya Y., Schmitto J., Berchtold-Herz M., Morshuis M., Shaw S.M., Saeed D., Lavee J., et al. Two-year outcome after implantation of a full magnetically levitated left ventricular assist device: Results from the ELEVATE Registry. Eur. Heart J. 2020;41:3801–3809. doi: 10.1093/eurheartj/ehaa639. [DOI] [PubMed] [Google Scholar]
- 11.Awad M., Czer L.S.C., Soliman C., Mirocha J., Ruzza A., Pinzas J., Rihbany K., Chang D., Moriguchi J., Ramzy D., et al. Prevalence of Warfarin Genotype Polymorphisms in Patients with Mechanical Circulatory Support. ASAIO J. 2015;61:391–396. doi: 10.1097/MAT.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehme A.K., Pamboukian S.V., George J.F., Beasley T.M., Kirklin J.K., Tallaj J., Dillon C., Levitan E.B., Griffin R., McGwin G., Jr., et al. Anticoagulation Control in Patients with Ventricular Assist Devices. ASAIO J. 2017;63:759–765. doi: 10.1097/MAT.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perera M.A., Cavallari L.H., Limdi N.A., Gamazon E.R., Konkashbaev A., Daneshjou R., Pluzhnikov A., Crawford D.C., Wang J., Liu N., et al. Genetic variants associated with warfarin dose in African-American individuals: A genome-wide association study. Lancet. 2013;382:790–796. doi: 10.1016/S0140-6736(13)60681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott S.A., Khasawneh R., Peter I., Kornreich R., Desnick R.J. Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics. 2010;11:781–791. doi: 10.2217/pgs.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limdi N.A., Beasley T.M., Crowley M.R., Goldstein J.A., Rieder M.J., Flockhart D.A., Arnett D.K., Acton R.T., Liu N. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African–Americans and European–Americans. Pharmacogenomics. 2008;9:1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z., Mondal N.K., Ding J., Koenig S.C., Slaughter M.S., Griffith B.P., Wu Z.J. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol. Cell Biochem. 2015;409:93–101. doi: 10.1007/s11010-015-2515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z., Koenig S.C., Slaughter M.S., Griffith B.P., Wu Z.J. Quantitative Characterization of Shear-Induced Platelet Receptor Shedding: Glycoprotein Ibalpha, Glycoprotein VI, and Glycoprotein IIb/IIIa. ASAIO J. 2018;64:773–778. doi: 10.1097/MAT.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles J.W., Wang H., Itakura H., Southwick A., Myers R.M., Iribarren C., Fortmann S.P., Go A.S., Quertermous T., Hlatky M.A. Association of polymorphisms in platelet and hemostasis system genes with acute myocardial infarction. Am. Heart J. 2007;154:1052–1058. doi: 10.1016/j.ahj.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feher G., Feher A., Pusch G., Lupkovics G., Szapary L., Papp E. The genetics of antiplatelet drug resistance. Clin. Genet. 2009;75:1–18. doi: 10.1111/j.1399-0004.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 20.Wurtz M., Kristensen S.D., Hvas A.M., Grove E.L. Pharmacogenetics of the Antiplatelet Effect of Aspirin. Curr. Pharm. Des. 2012;18:5294–5308. doi: 10.2174/138161212803251907. [DOI] [PubMed] [Google Scholar]
- 21.Alrashid M.H., Al-Serri A., Alshemmari S.H., Koshi P., Al-Bustan S.A. Association of Genetic Polymorphisms in the VKORC1 and CYP2C9 Genes with Warfarin Dosage in a Group of Kuwaiti Individuals. Mol. Diagn Ther. 2016;20:183–190. doi: 10.1007/s40291-016-0190-7. [DOI] [PubMed] [Google Scholar]
- 22.Limdi N.A., Veenstra D.L. Warfarin pharmacogenetics. Pharmacotherapy. 2008;28:1084–1097. doi: 10.1592/phco.28.9.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishna Kumar D., Shewade D.G., Loriot M.A., Beaune P., Balachander J., Sai Chandran B.V., Adithan C. Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur. J. Clin. Pharmacol. 2014;70:47–56. doi: 10.1007/s00228-013-1581-x. [DOI] [PubMed] [Google Scholar]
- 24.Agúndez J.A.G., Martínez C., Pérez-Sala D., Carballo M., Torres M.J., García-Martín E. Pharmacogenomics in Aspirin Intolerance. Curr. Drug Metab. 2009;10:998–1008. doi: 10.2174/138920009790711814. [DOI] [PubMed] [Google Scholar]
- 25.Wadelius M., Chen L.Y., Eriksson N., Bumpstead S., Ghori J., Wadelius C., Bentley D., McGinnis R., Deloukas P. Association of warfarin dose with genes involved in its action and metabolism. Hum. Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hylckama Vlieg A., Baglin C.A., Bare L.A., Rosendaal F.R., Baglin T.P. Proof of principle of potential clinical utility of multiple SNP analysis for prediction of recurrent venous thrombosis. J. Thromb. Haemost. 2008;6:751–754. doi: 10.1111/j.1538-7836.2008.02920.x. [DOI] [PubMed] [Google Scholar]
- 27.Shi H., Yang S., Liu Y., Huang P., Lin N., Sun X., Yu R., Zhang Y., Qin Y., Wang L. Study on Environmental Causes and SNPs of MTHFR, MS and CBS Genes Related to Congenital Heart Disease. PLoS ONE. 2015;10:e0128646. doi: 10.1371/journal.pone.0128646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie M.D., Hahn L.W., Roodi N., Bailey L.R., Dupont W.D., Parl F.F., Moore J.H. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am. J. Hum. Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winham S.J., Motsinger-Reif A.A. An R package implementation of multifactor dimensionality reduction. BioData Min. 2011;4:1–8. doi: 10.1186/1756-0381-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patron J., Serra-Cayuela A., Han B., Li C., Wishart D.S. Assessing the performance of genome-wide association studies for predicting disease risk. PLoS ONE. 2019;14:e0220215. doi: 10.1371/journal.pone.0220215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Eitan L.N., Almasri A.Y., Khasawneh R.H. Effects of CYP2C9 and VKORC1 polymorphisms on warfarin sensitivity and responsiveness during the stabilization phase of therapy. Saudi Pharm. J. 2019;27:484–490. doi: 10.1016/j.jsps.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topkara V.K., Levin A., Mody K., Garan A., Ronquillo K.O., Tiburcio M., Murphy J.S., Parkis G., Takeda K., Takayama H., et al. Association of Warfarin Genotype with Thrombosis and Bleeding Events in Continuous-Flow Left Ventricular Assist Device (CF-LVAD) Patients. J. Heart Lung Transpl. 2015;34:S13. doi: 10.1016/j.healun.2015.01.022. [DOI] [Google Scholar]
- 33.Hu J., Mondal N.K., Sorensen E.N., Cai L., Fang H.B., Griffith B.P., Wu Z.J. Platelet glycoprotein Ibalpha ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J. Heart Lung Transpl. 2014;33:71–79. doi: 10.1016/j.healun.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z., Zhang J., Kareem K., Tran D., Conway R.G., Arias K., Griffith B.P., Wu Z.J. Device-induced platelet dysfunction in mechanically assisted circulation increases the risks of thrombosis and bleeding. Artif. Organs. 2019;43:745–755. doi: 10.1111/aor.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koliopoulou A., McKellar S.H., Rondina M., Selzman C.H. Bleeding and thrombosis in chronic ventricular assist device therapy: Focus on platelets. Curr. Opin. Cardiol. 2016;31:299–307. doi: 10.1097/HCO.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grinshtein Y.I., Kosinova A.A., Grinshtein I.Y., Subbotina T.N., Savchenko A.A. The Prognostic Value of Combinations of Genetic Polymorphisms in the ITGB3, ITGA2, and CYP2C19*2 Genes in Predicting Cardiovascular Outcomes After Coronary Bypass Grafting. Genet. Test. Mol. Biomark. 2018;22:259–265. doi: 10.1089/gtmb.2017.0177. [DOI] [PubMed] [Google Scholar]
- 37.Stankovic S., Majkic-Singh N. Genetic aspects of ischemic stroke: Coagulation, homocysteine, and lipoprotein metabolism as potential risk factors. Crit. Rev. Clin. Lab. Sci. 2010;47:72–123. doi: 10.3109/10408361003791520. [DOI] [PubMed] [Google Scholar]
- 38.Kucharska-Newton A.M., Monda K.L., Campbell S., Bradshaw P.T., Wagenknecht L.E., Boerwinkle E., Wasserman B.A., Heiss G. Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2011;216:151–156. doi: 10.1016/j.atherosclerosis.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grove E.L., Orntoft T.F., Lassen J.F., Jensen H.K., Kristensen S.D. The platelet polymorphism PlA2 is a genetic risk factor for myocardial infarction. J. Int. Med. 2004;255:637–644. doi: 10.1111/j.1365-2796.2004.01327.x. [DOI] [PubMed] [Google Scholar]
- 40.Nadasi E., Bene J., Havasi V., Komlosi K., Talian G., Melegh G., Papp E., Gasztonyi B., Szolnoki Z., Sandor J., et al. Detection of the Leu40Arg variant of the platelet glycoprotein IIb/IIIa receptor in subjects with thrombotic diseases. Thromb. Res. 2005;116:479–482. doi: 10.1016/j.thromres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Lanni F., Santulli G., Izzo R., Rubattu S., Zanda B., Volpe M., Iaccarino G., Trimarco B. The PlA1/A2 polymorphism of glycoprotein IIIa and cerebrovascular events in hypertension: Increased risk of ischemic stroke in high-risk patients. J. Hypertens. 2007;25:551–556. doi: 10.1097/HJH.0b013e328013cd67. [DOI] [PubMed] [Google Scholar]
- 42.van Oijen M.G., Huybers S., Peters W.H., Drenth J.P., Laheij R.J., Verheugt F.W., Jansen J.B. Polymorphisms in genes encoding acetylsalicylic acid metabolizing enzymes are unrelated to upper gastrointestinal health in cardiovascular patients on acetylsalicylic acid. Br. J. Clin. Pharmacol. 2005;60:623–628. doi: 10.1111/j.1365-2125.2005.02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maruo Y., Iwai M., Mori A., Sato H., Takeuchi Y. Polymorphism of UDP-Glucuronosyltransferase and Drug Metabolism. Curr. Drug Metab. 2005;6:91–99. doi: 10.2174/1389200053586064. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Kuehl G.E., Bigler J., Rimorin C.F., Schwarz Y., Shen D.D., Lampe J.W. UGT1A6 polymorphism and salicylic acid glucuronidation following aspirin. Pharmacogen. Genom. 2007;17:571–579. doi: 10.1097/01.fpc.0000236339.79916.07. [DOI] [PubMed] [Google Scholar]
- 45.Sheth H., Northwood E., Ulrich C.M., Scherer D., Elliott F., Barrett J.H., Forman D., Wolf C.R., Smith G., Jackson M.S., et al. Interaction between polymorphisms in aspirin metabolic pathways, regular aspirin use and colorectal cancer risk: A case-control study in unselected white European populations. PLoS ONE. 2018;13:e0192223. doi: 10.1371/journal.pone.0192223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C., He Y., Shan K.R., Tan K., Zhang T., Wang C.J., Guan Z.Z. Correlations between polymorphisms in the uridine diphosphate-glucuronosyltransferase 1A and C-C motif chemokine receptor 5 genes and infection with the hepatitis B virus in three ethnic groups in China. J. Int. Med. Res. 2018;46:739–751. doi: 10.1177/0300060517730174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherer D., Koepl L.M., Poole E.M., Balavarca Y., Xiao L., Baron J.A., Hsu L., Coghill A.E., Campbell P.T., Kleinstein S.E., et al. Genetic variation in UGT genes modify the associations of NSAIDs with risk of colorectal cancer: Colon cancer family registry. Genes Chromosomes Cancer. 2014;53:568–578. doi: 10.1002/gcc.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson C.L., Plummer S.J., Merkulova A., Cheng I., Tucker T.C., Casey G., Li L. No association between cyclooxygenase-2 and uridine diphosphate glucuronosyltransferase 1A6 genetic polymorphisms and colon cancer risk. World J. Gastroenterol. 2009;15:2240–2244. doi: 10.3748/wjg.15.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iskakova A.N., Romanova A.A., Aitkulova A.M., Sikhayeva N.S., Zholdybayeva E.V., Ramanculov E.M. Polymorphisms in genes involved in the absorption, distribution, metabolism, and excretion of drugs in the Kazakhs of Kazakhstan. BMC Genet. 2016;17:23. doi: 10.1186/s12863-016-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle A.J., Jorde U.P., Sun B., Park S.J., Milano C.A., Frazier O.H., Sundareswaran K.S., Farrar D.J., Russell S.D. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: An analysis of more than 900 HeartMate II outpatients. J. Am. Coll. Cardiol. 2014;63:880–888. doi: 10.1016/j.jacc.2013.08.1656. [DOI] [PubMed] [Google Scholar]
- 51.Xiang Q., Ji S.D., Zhang Z., Zhao X., Cui Y.M. Identification of ITGA2B and ITGB3 Single-Nucleotide Polymorphisms and Their Influences on the Platelet Function. BioMed. Res. Int. 2016;2016:5675084. doi: 10.1155/2016/5675084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sciaccaluga C., Ghionzoli N., Mandoli G.E., D’Ascenzi F., Focardi M., Valente S., Cameli M. Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence. Biomolecules. 2022;12:334. doi: 10.3390/biom12020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oussalah A., Bosco P., Anello G., Spada R., Gueant-Rodriguez R.M., Chery C., Rouyer P., Josse T., Romano A., Elia M., et al. Exome-Wide Association Study Identifies New Low-Frequency and Rare UGT1A1 Coding Variants and UGT1A6 Coding Variants Influencing Serum Bilirubin in Elderly Subjects: A Strobe Compliant Article. Medicine. 2015;94:e925. doi: 10.1097/MD.0000000000000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazur-Kominek K., Romanowski T., Bielawski K., Kielbratowska B., Preis K., Domzalska-Popadiuk I., Slominska-Fraczek M., Sznurkowska K., Renke J., Plata-Nazar K., et al. Association between uridin diphosphate glucuronosylotransferase 1A1 (UGT1A1) gene polymorphism and neonatal hyperbilirubinemia. Acta Biochim. Pol. 2017;64:351–356. doi: 10.18388/abp.2016_1450. [DOI] [PubMed] [Google Scholar]
- 55.Justyna Gil J., Sąsiadek M.M. Gilbert syndrome: The UGT1A1*28 promoter polymorphism as a biomarker of multifactorial diseases and drug metabolism. Biomark. Med. 2012;6:223–230. doi: 10.2217/bmm.12.4. [DOI] [PubMed] [Google Scholar]
- 56.Sarajlic P., Simonsson M., Jernberg T., Back M., Hofmann R. Incidence, associated outcomes, and predictors of upper gastrointestinal bleeding following acute myocardial infarction: A SWEDEHEART-based nationwide cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2021:1–9. doi: 10.1093/ehjcvp/pvab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
National Laboratory Astana (contact via phone or mail) for researchers who meet the criteria for access to confidential data. The data underlying the results presented in the study are available from the authors, phone number: +77172706501, mail: akilzhanova@nu.edu.kz.