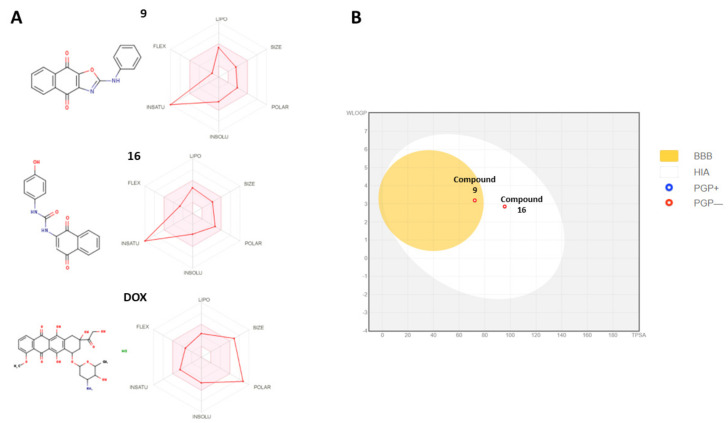
Figure 8.
The bioavailability properties of the most potent compounds, 9 and 16, and their comparison to doxorubicin (DOX). Panel (A) demonstrates the drug-likeness properties of compounds 9 and 16. The pink area represents the optimal range for each Lipinski drug-like properties (lipophilicity: XLOGP3 between −0.7 and +5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å2, solubility: log S not higher than 6, saturation: fraction of carbons in the sp3 hybridization not less than 0.25, and flexibility: no more than 9 rotatable bonds). Panel (B) demonstrates the passive gastrointestinal absorption (HIA) and blood–brain permeability parameters. The red dots indicate that compounds 9 and 16 are not predicted to be substrates of P-gp transporter. Doxorubicin is not represented in this graph due to estimated off-limit values.