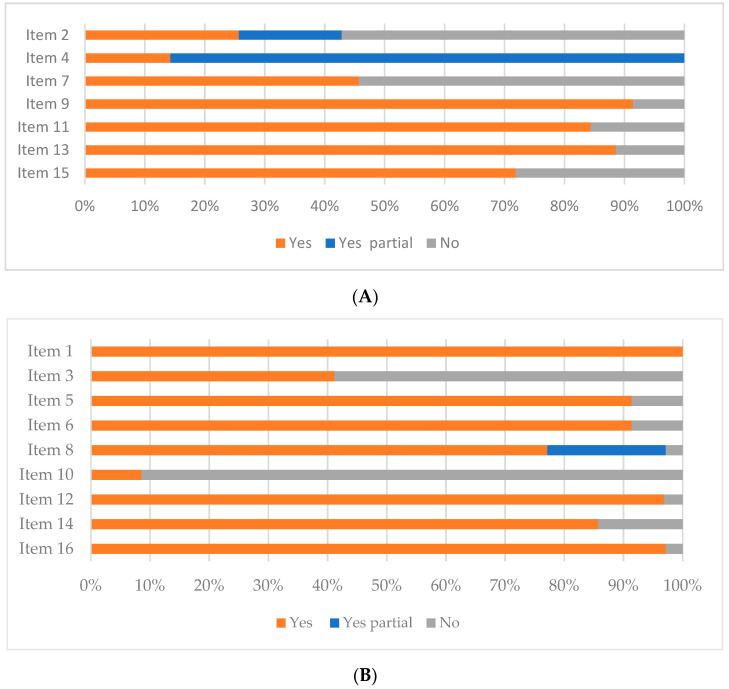
Figure 2.
Accomplishment of systematic review with AMSTAR-2 items. (A) Critical items. (B) Non-critical items. Critical items: Item 2: Previous protocol review; Item 4: Adequate literature search; Item 7: Excluded studies justification; Item 9: Bias risk of individual studies included; Item 11: Appropriate meta-analysis methods; Item 13: Consideration of the bias risk in the interpretation of the review results; Item 15: Assessment of the presence and probable impact of publication bias. Non-critical items: Item 1: Research questions and inclusion criteria include PICO components; Item 3: Explaining decision about the study designs to include in the review; Item 5: Study selection performed in duplicate; Item 6: Data extraction performed in duplicate; Item 8: Describing included studies with sufficient detail; Item 10: Reporting the sources of funding for the studies included in the review; Item 12: Assessing the potential impact of bias risk on results; Item 14: Satisfactory explanation and discussing any observed heterogeneity in the review results; and Item 16: Potential sources of conflict including any funding received.