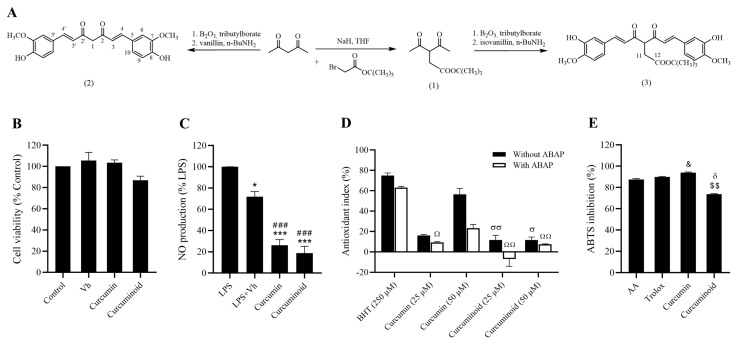
Figure 1.
Representative scheme of the chemical synthesis of curcumin (2) and curcuminoid (3) are shown (A). Curcumin and curcuminoid (3) demonstrate a strong anti-inflammatory potential in vitro. Cell viability was evaluated in Cos-7 cells by the Alamar blue assay (B) and the anti-inflammatory effect was assessed in Raw 264.7 macrophages through the Griess reagent method (C). Antioxidant activity was evaluated by TBARS (D) and ABTS assays (E). Results are expressed as mean ± S.E.M.; vertical bars represent S.E.M.; statistical differences were evaluated by Tukey’s test. * vs. LPS; # vs. LPS + Vh; & vs. AA; δ vs. Trolox; $ vs. Curcumin; σ vs. BHT (250 µM) without ABAP; Ω vs. BHT (250 µM) with ABAP. 1 symbol p < 0.05; 2 symbol p < 0.01; 3 symbol p < 0.001.