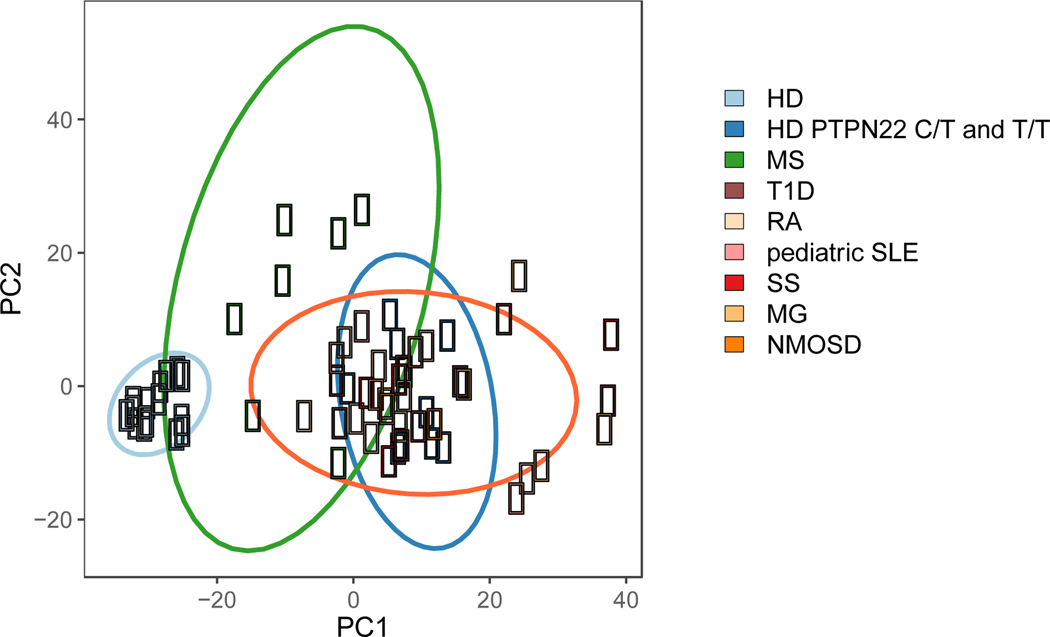
Figure 3. Principal component analysis of the frequency of polyreactive and autoreactive B cells in seven distinct autoimmune diseases, healthy donors carrying 1858T PTPN22 risk allele(s) and non-carrier healthy donors.
Proportions of polyreactive antibodies expressed by new emigrant/transitional B cells and proportions of both polyreactive and autoreactive antibodies expressed by mature naïve B cells were plotted for each subject group. Healthy donor (HD)-derived B cells populations (light blue) cluster together, reflecting the low frequencies of polyreactive and autoreactive clones correlating with functional early B cell tolerance checkpoints. Type 1 diabetes (T1D), rheumatoid arthritis (RA), pediatric systemic lupus erythematosus (SLE), Sjögren’s syndrome (SjS), myasthenia gravis (MG), and neuromyelitis optica spectrum disease (NMOSD) segregate away from healthy donors, illustrating the accumulation of polyreactive and autoreactive B cells due to impaired central and peripheral B cell tolerance checkpoints. Healthy donors with either a heterozygous (C/T) or homozygous (T/T) PTPN22 polymorphism (dark blue), who also display defective early B cell tolerance checkpoints, cluster with the autoimmune disease cohort. B cells from MS patients (green) display a heterogeneous pattern. While two patients with MS cluster with the autoimmune cohort and displayed impaired central and peripheral B cell tolerance checkpoints, most MS patients cluster as an independent group characterized by specific defects of the peripheral B cell tolerance checkpoint.