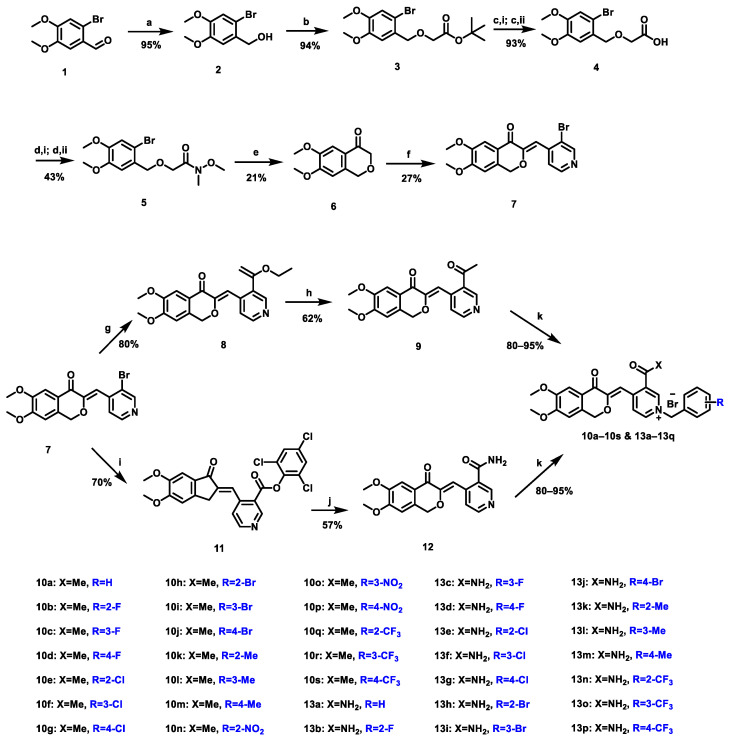
Scheme 1.
Reagents and conditions: (a) NaBH4, MeOH, 0 °C, 20 min; (b) tert-butyl bromoacetate, tetrabutylammonium bromide, KOH in H2O, toluene, 50 °C, 1 h; (c,i) MeONa, MeOH, r.t., 15 min; (c,ii)H2O, r.t., 5 min; then Conc. HCl, r.t., 5 min; (d,i) oxalyl chloride, DMF, dry DCM, r.t., 20 min; (d,ii) N,O-dimethylhydroxylamine hydrochloride, K2CO3, dry MeCN, r.t., 30 min; (e) t-BuLi, dry THF, −78 °C, 10 min; (f) 3-bromo-4-pyridinecarboxaldehyde, p-toluenesulfonic acid monohydrate, toluene, reflux, 4 h; (g) Pd(dba)2, Ph3P, tributyl(1-ethoxyvinyl)stannane, toluene, 110 °C, 10 h; (h) 6 N HCl, H2O, 50 °C, 1 h; (i) Xantphos, Pd(OAc)2, Et3N in toluene, 2,4,6-trichlorophenyl formate, dry toluene, 110 °C, 10 h; (j) 0.4 M NH3 in dioxane, 80 °C, 17 h; (k) benzyl bromides with different substituents, dry MeCN, 85 °C, 1–3 h.