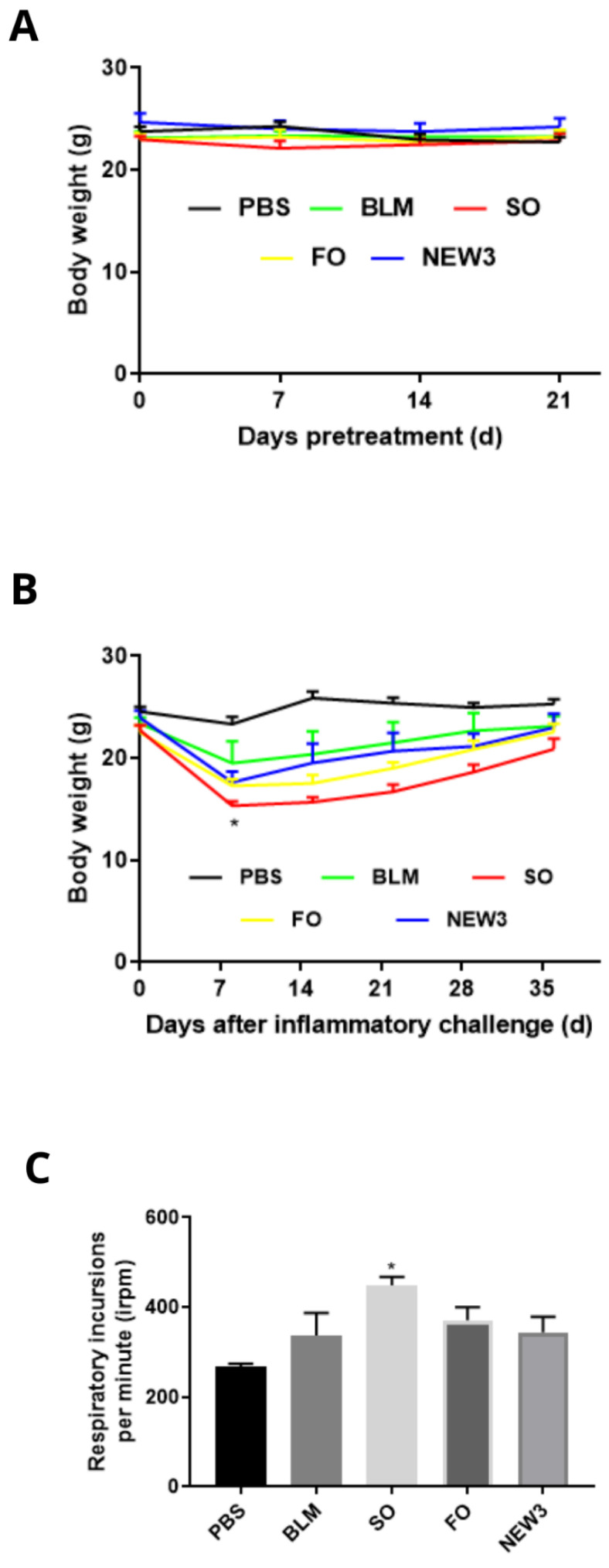
Figure 3.
Pulmonary clinical aspects. (A) Body weight of mice during pre-treatment over 21 days. (n = 8). Legend: PBS: negative control group; BLM: positive control group; SO: group pretreated with sunflower oil (100 mg/kg); FO: animals pretreated with fish oil (100 mg/kg); NEW3: animals pretreated with fish oil nanoemulsion (100 mg/kg). (B) Body weight of mice over 36 days after pulmonary inflammatory challenge (n = 8). Legend: PBS: negative control group; BLM: positive control group challenged with BLM (5 mg/mL); SO: group pretreated with sunflower oil (100 mg/kg) and challenged with BLM (5 mg/mL); FO: group pretreated with fish oil (100 mg/kg) and challenged with BLM (5 mg/mL); NEW3: group pretreated with fish oil nanoemulsion (100 mg/kg) and challenged with BLM (5 mg/mL). Data are represented as mean ± standard deviation. Values were submitted to Tukey’s multiple comparison tests; * represents the statistically significant difference (p < 0.05), compared to the other experimental groups. (C) Respiratory rate on the 29th day after inflammatory challenge (n = 8). Caption: Irpm: respiratory incursions per minute. PBS: negative control group; BLM: positive control group challenged with BLM (5 mg/mL); SO: group pretreated with sunflower oil (100 mg/kg) and challenged with BLM (5 mg/mL); FO: group pretreated with fish oil (100 mg/kg) and challenged with BLM (5 mg/mL); NEW3: group pretreated with fish oil nanoemulsion (100 mg/kg) and challenged with BLM (5 mg/mL). Data are represented as mean ± standard deviation. Values were submitted to Tukey’s multiple comparison tests; * represents the statistically significant difference (p < 0.05), compared to the PBS group.