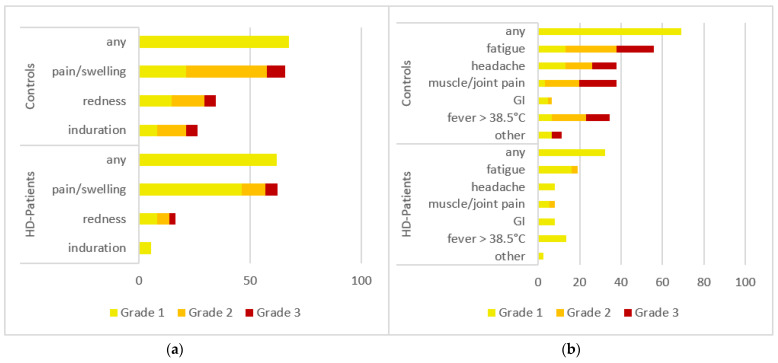
Figure 5.
Adverse events (AEs) in controls (top) and HD patients (bottom). The AEs were recorded using a standardized questionnaire and graded by the patients (Grade 1: mild, does not interfere with activity; Grade 2: moderate, interferes with activity; Grade 3: severe, prevents daily activity). No Grade 4 events (emergency department visits or hospitalization) were reported. HD patients, patients on haemodialysis. All numbers represent the percentages of dialysis (n = 36) and control (n = 61) patients. (a) local AEs after the third vaccination; (b) Systemic AEs after the third vaccination.