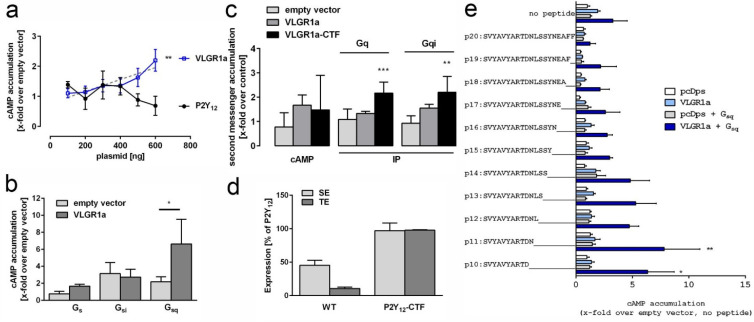
Figure 2.
Signal transduction and activation of VLGR1. (a) HEK293T cells were transfected with increasing amounts (100–600 ng/well) of plasmids encoding either human VLGR1 or human P2Y12. VLGR1, but not P2Y12, a known Gi coupling receptor, caused a dose-dependent increase of cAMP. Statistics displayed significant linear regression for VLGR1. (b) Coupling analysis of VLGR1a using chimeric G proteins in cAMP accumulation assay revealed significant 2nd messenger accumulation with a chimera that couples to a Gq-binding receptor. (c) Comparison of basal 2nd messenger production of full length and CTF, where the NTF was replaced with the N-terminus of P2Y12 to ensure proper surface expression of the mutant. Constitutive activity of the CTF is observed in IP accumulation assay with and without a Gqi chimeric protein, indicating coupling to Gq and Gi. (d) Cell surface (SE) and total cell expression (TE) of full length and CTF mutant constructs of VLGR1 in relation to P2Y12 (100%), which served as positive control. (e) Screening for VLGR1 Stachel-mimicking peptides. A Stachel-derived peptide library of VLGR1 was tested in cAMP with and without the addition of a Gsq chimeric G protein. Peptides 10 and 11 amino acids long were found to significantly activate VLGR1 in comparison to pcDps when the Gsq chimera was added. (a–e): Data are given as means ± S.D. of three independent experiments each performed in triplicate. (b,c): Statistics was performed using one-way ANOVA with Bonferroni as post-hoc test and (d) utilized one-way ANOVA with Sidak’s multiple comparison test. p-values in b, c and e: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.