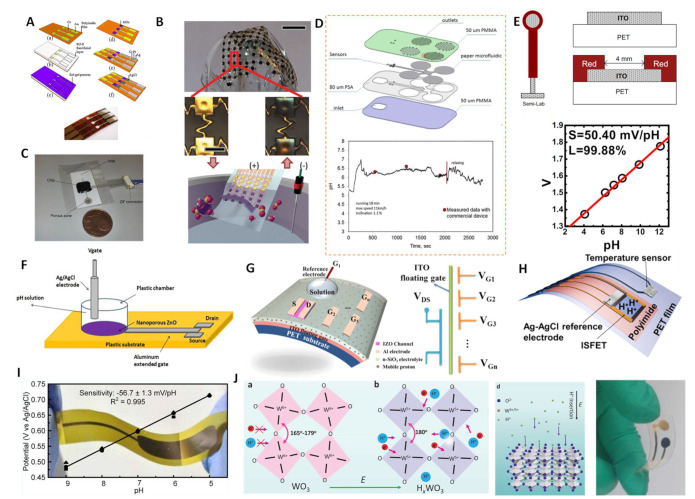
Figure 6.
MOx–based wearable pH sensors. (A) The preparation process for IrOx–based flexible pH sensors. Reprinted with permission from [52], Copyright (2011) Elsevier. (B) Picture of thin compliant array of pH sensors. Reprinted with permission from [37], Copyright (2014) John Wiley and Sons publications. (C) Integrated silicon chip (with paper at the inlet for absorbing experiments). Reprinted with permission from [110], Copyright (2016) Elsevier. (D) Schematic representation of the fabrication steps of the micro-fluidic chip. The figure shows the real-time monitoring image of the human sweat pH value from the microfluidic chip. Reprinted with permission from [111], Copyright (2017) Elsevier. (E) Schematic and cross–section of the ITO/PET electrode. The figure shows the pH linear response curve. Reprinted with permission from [112], Copyright (2012) Elsevier. (F) Flexible pH sensor based on extended gate transistor. Reprinted with permission from [97], Copyright (2014) Elsevier. (G) Flexible pH sensor based on an IZO neuromorphic transistor with multiple gate electrodes. Reprinted with permission from [51], Copyright (2015) Spring Nature. (H) A wearable ion-sensitive filed effect transistor (ISFET) integrating flexible pH and temperature devices. Reprinted with permission from [113], Copyright (2017) American Chemistry Society. (I) Flexible WO3–based pH sensor on metal substrate with −56.7 ± 1.3 mV/pH sensitivity. Reprinted with permission from [93], Copyright (2014) American Chemistry Society. (J) Structures of WO3 before and after proton intercalation (HxWO3). The image on the right shows a schematic of the insertion of a proton into the lattice of WO3 and the photograph on the left shows the flexible electrode after proton intercalation. Reprinted with permission from [109], Copyright (2022) John Wiley and Sons publications.