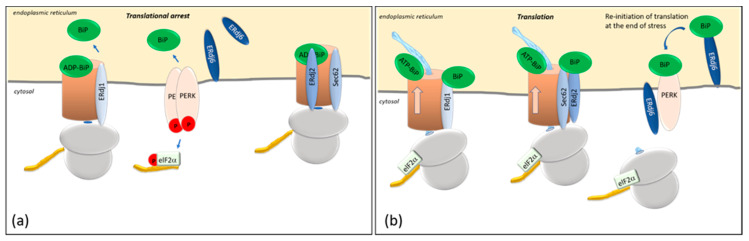
Figure 1.
(a) At the translocon (brown cylinder), the pore is gated by BiP, which, in its ADP-bound form, closes the gate and opens it in its ATP-bound form. Translational arrest occurs when BiP dissociates from the luminal domains of ERdj1, ERdj2, and PERK. The release of BiP from PERK results in the dimerization of PERK, its autophosphorylation, and the subsequent phosphorylation of eIF2α (p-eIF2α), which inhibits eIF2α-dependent translation. (b) The translation of proteins is controlled by the co-chaperones ERdj1, ERdj2/Sec62, and ERdj6. In their BiP-bound forms, ERdj1 and ERdj2/Sec62 enable protein synthesis. The silencing of PERK and the PERK-signaling pathway occur by the binding of BiP to its luminal domain, which results in the release of eIF2α-dependent translation and protein synthesis. BiP, immunoglobin binding protein; PERK, protein kinase RNA-like endoplasmic reticulum kinase; eIF2α, alpha subunit of eukaryotic initiation factor 2.