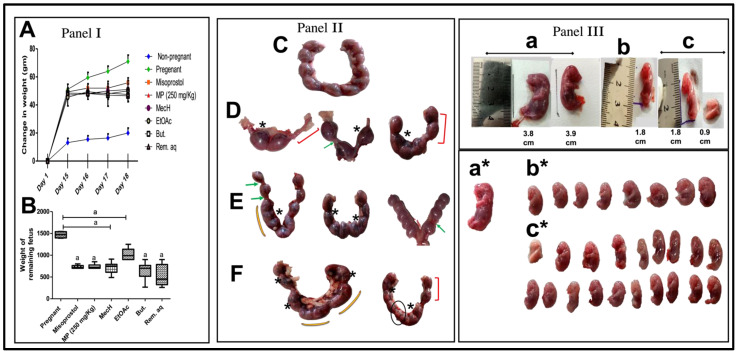
Figure 2.
(A) change in weight of pregnant rats in different groups as compared to unmated/unpregnant female through the study (days: 1st, 15th, 16th, 17th, and 18th of gestation), and (B) weight of remaining fetus (Panel I). (A) Two-Way RM ANOVA with one factor repetition (time: day) was utilized to analyze the change in rat weight and (B) the non-parametric data of fetal weight were expressed as the median (min-max) and were analyzed using the non-parametric Kruskal–Wallis followed by Dunn’s as the post hoc test and student t test (Panel I). Common pathological observations and features of uterine tissues. (C) Pregnant rats with normal fetal distribution in the two horns; (D–F) MP (extract/fraction) or misoprostol on the 18th day of gestation. Observations: (D); the uterus with asymmetrical distribution of fetuses in the two horns, complete absence of fetus in one horn, remarkable resorption sites, and reduced number of fetal balls, (E,F); asymmetrical distribution of fetuses in the two horns, absence of boundaries between fetal balls, reduced sized fetal balls, notable resorption sites, and intensive bleeding. The asymmetric distribution of fetuses in the two uterine horns: red brackets; resorption points: green arrows; hematoma: star; and absence of boundaries between fetal balls: yellow arc; reduced fetal balls: black circle (Panel II). MP (extract or fractions) and Misoprostol-mediated structural abnormalities in fetus including height, weight, and physical appearance; (a,a*) pregnant, (b,b*) Misoprostol, and (c,c*) MP; extract or fractions groups respectively (Panel III).