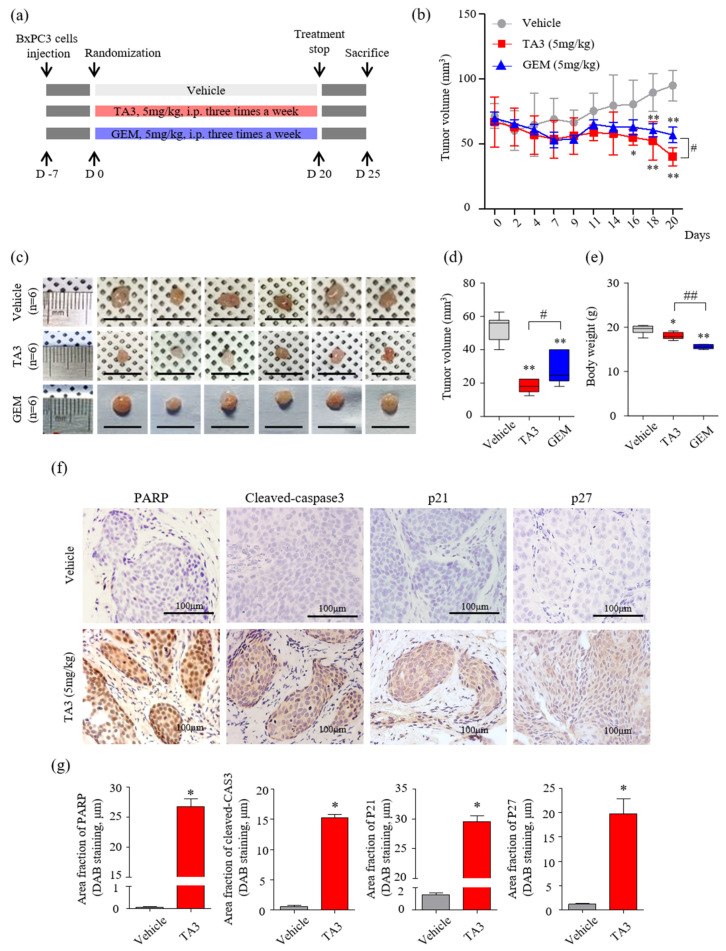
Figure 5.
Effects of TA3 on pancreatic cancer growth in a xenograft mouse model. (a) Experimental methods of schematic representation as described in the Materials and Methods section. Briefly, BxPC-3 cells (1 × 107 cells/mouse) were mixed with Matrigel at a ratio of 1:1 (v/v) before subcutaneous injection and randomly assigned to six mice of each group. Then, 100 μL of PBS and each drug (5 mg/kg of TA3 and GEM) were intraperitoneally injected into the mice of each group three times a week for 20 days with a measure of tumor volume and euthanized five days after the end of treatment. (b) Tumor volume was monitored for three weeks after treatment with TA3 (5 mg/kg). (c) Tumors harvested from each treatment group were photographed. (d) The volume of the dissected primary tumor was calculated using the formula V = 4/3 πr3. (e) Estimated cytotoxicity using the measured body weight. (f) Paraffin-embedded tumor sections were stained brown through immunoreactivity with PARP, cleaved caspase-3, p21, and p27 antibodies using immunohistochemical analysis. (g) Comparison between the immunostaining (DAB) area fraction of PARP, cleaved caspase-3, p21, and p27 in paraffin-embedded tumor sections. All box plot presented the mean ± SD (* p < 0.05, ** p < 0.01, vs. vehicle; # p < 0.05, ## p < 0.01 comparison of TA3 and GEM; n = 6/group).