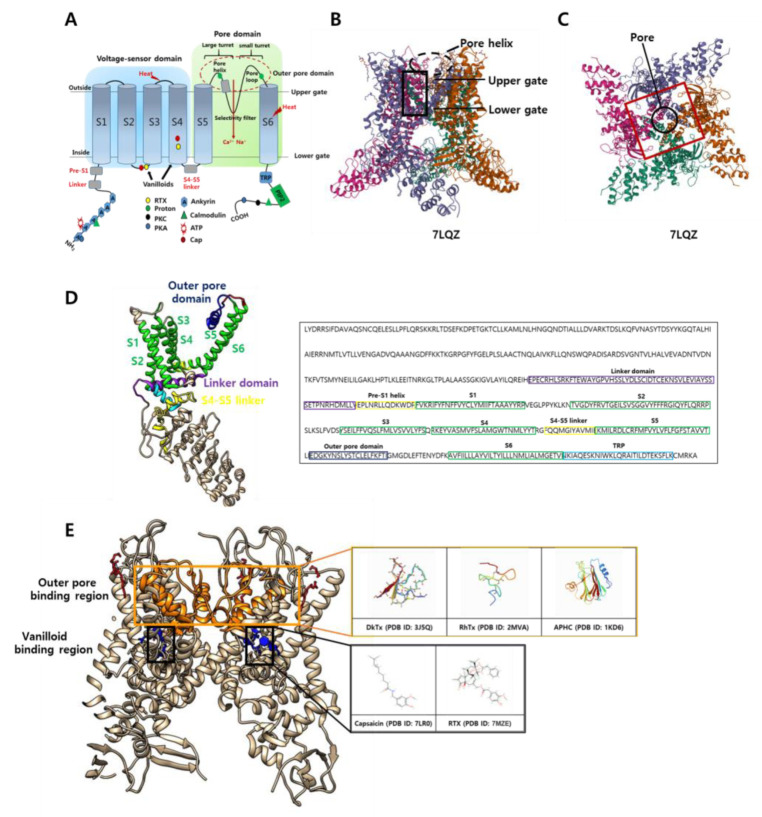
Figure 1.
The structure and binding sites of the transient receptor potential vanilloid 1 (TRPV1) channel. (A) TRPV1 is a homotetrameric transmembrane protein containing a voltage-sensory domain, pore domain, S4–5 helix linker, and TRP box. The N-terminal end comprises ankyrin repeats and a calmodulin interaction site, while the C-terminal end contains a PIP2 interaction site. Amino acid residues at both ends can be phosphorylated by PKC and PKA. Vanilloid agonist sites are located in the S2–4 transmembrane domain. Both heat- and protein-initiated stimuli are mediated by specific residues located in the extracellular membrane domain (or loops). The selectivity filter is formed by the loop connecting the pore helix and S6 helix. The large and small turret subunits are connected to the S5 and S6 domains. The outer pore domain is indicated by a red dashed circle. (B) Side view of the TRPV1 structure: the tetrameric structure of the pore helix and the upper and lower gates, which regulate channel activation. The vanilloid pocket region is highlighted by the black box, and the pore helix is indicated by a black dashed circle (PBD ID: 7LQZ). (C) Top view of the TRPV1 structure: the outer pore region is highlighted by a red box, and the pore region is indicated by a black circle (PBD ID: 7LQZ). The symbols beneath each 3D structure are the access numbers in the Protein Data Bank (PDB). (D) Side view of a single TRPV1 subunit color coded as described in B (PDB ID: 7L2S). Sequence alignment of rat TRPV1 construct (NW_024405602); linker domain, pre-S1 helix (linker), S1–6, outer pore domain, and TRP are highlighted in purple, yellow, green, dark blue, and light blue, respectively. (E) Side view of the TRPV1 tetrameric structure (PDB ID: 7L2S) showing the outer pore binding venom peptides (DkTx, RhTx, and APHC) and their sites (orange box). Similarly, vanilloid binding agonist (capsaicin and RTX) and its site (black box) are shown.