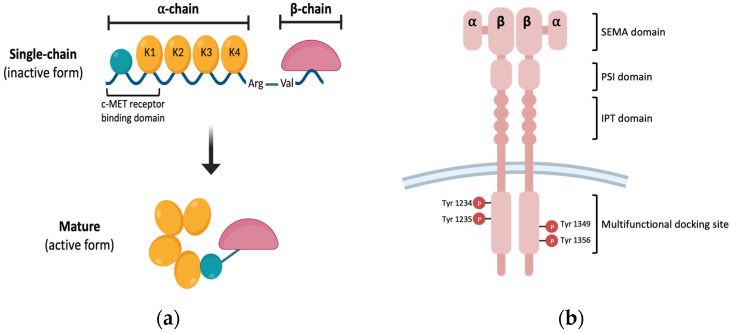
Figure 1.
Schematic structure of HGF and MET. (a) HGF structure. HGF is a heterodimer that consists of alpha- and beta-chains linked via disulfide bonds. An alpha chain is composed of N-terminal hairpin domain and four kringle domains, and a beta chain is composed of serine-protease homology domain. Alpha- and beta-chains are connected by disulfide bonds and are cleaved by serum-derived proteases to convert to the active from. (b) MET structure. c-MET is a heterodimer linked by an extracellular alpha-chain and a transmembrane beta-chain. The beta-chain consists of a SEMA domain, PSI domain, IPT domain, multifunctional docking site, and C-terminal tail region. The multifunctional docking site has several tyrosine kinase domains.