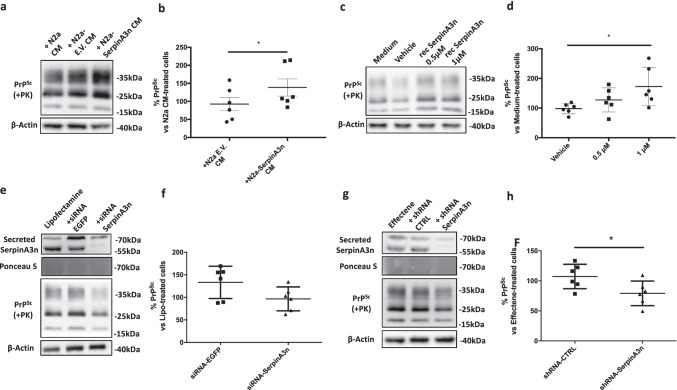
Fig. 7.
SerpinA3n modulation alters PrPSc level in ScN2a RML cells. a, c Representative WB image of PrPSc in lysates from ScN2a RML treated with CM from N2a, N2a-EV, and N2a-SerpinA3n (a) or treated with recombinant SerpinA3n (0.5 µM and 1 µM), vehicle (10 mM Tris–HCl, 50 mM KCl, and pH 8.0), and medium alone (c). β-actin was used as protein loading control. PrPSc signal was developed on another membrane after PK-digestion of cell lysates. b, d Densitometric analysis of β-actin-normalized PrPSc levels in N2a-EV and N2a-SerpinA3n CM–treated ScN2a RML relative to cell treated with CM from N2a (b, n = 6) or in recombinant SerpinA3n and vehicle-treated N2a relative to cell treated with medium only (d, n = 6). Statistical significance was performed by the Wilcoxon matched pairs signed rank test (b) or by the Friedman test with Dunn’s multiple comparisons test (d), *p < 0.05. e, g Representative WB image of PrPSc in lysates from ScN2a RML transfected with siRNA-SerpinA3n (e) and shRNA-SerpinA3n (g). β-actin and Ponceau staining were used as protein loading control. PrPSc signal was developed on another membrane after PK-digestion of cell lysates. f, h Densitometric analysis of β-actin-normalized PrPSc levels in siRNA-EGFP and siRNA-SerpinA3n–transfected cells relative to ScN2a RML cells transfected with Lipofectamine only (f, n = 6) or in shRNA-CTRL and shRNA-Serpina3n–transfected cells relative to ScN2a RML cells transfected with Effectene only (g, n = 6). Statistical significance was performed by the Wilcoxon matched pairs signed rank test, *p < 0.05