Figure 40.
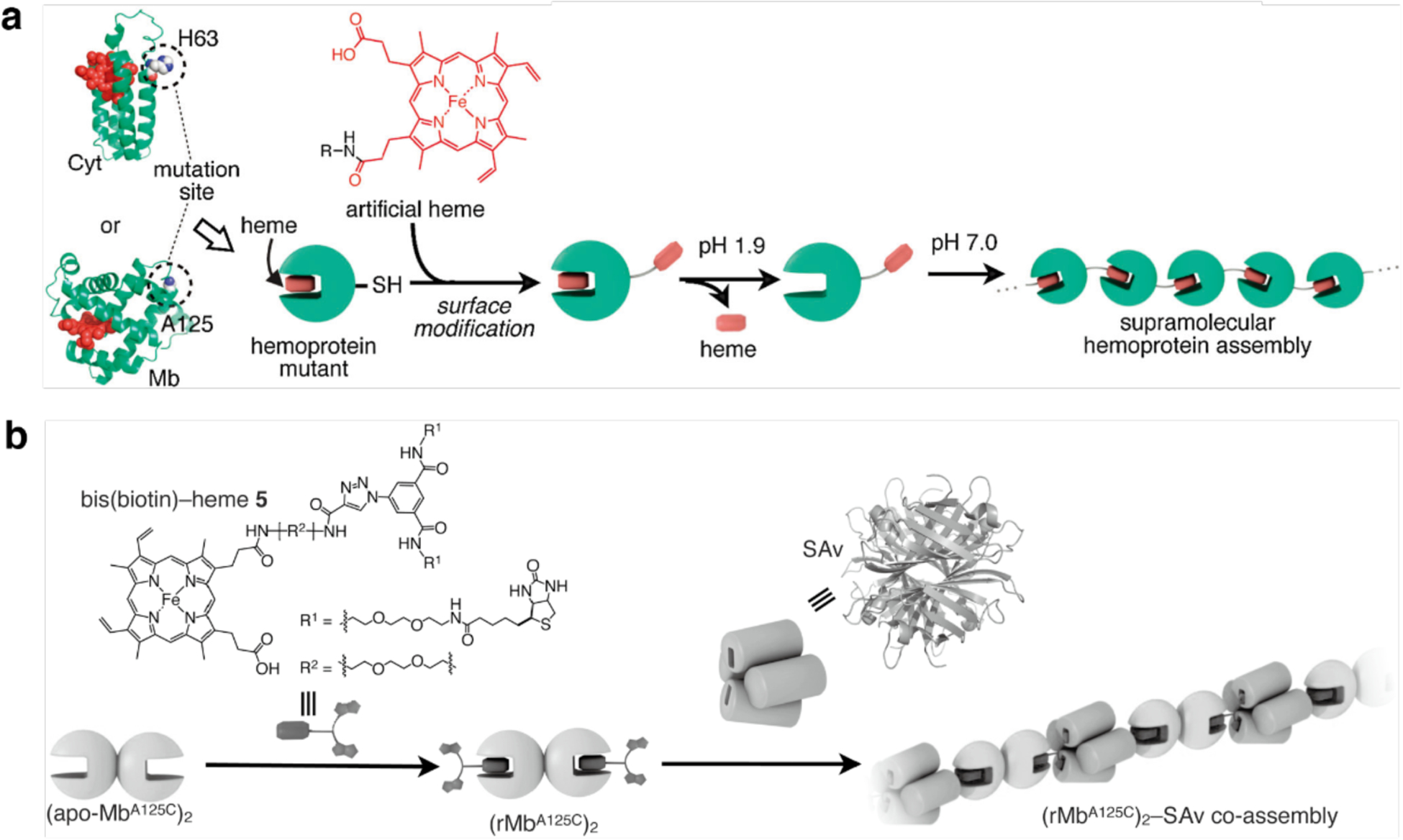
Fibrillar protein self-assembly through apo-hemoprotein/heme interactions. a) H63C and A125C mutations are incorporated into cytochrome b562 (Cyt) and myoglobin (Mb) respectively to position Cys residues on the protein surface for further conjugation to artificial heme derivatives. Under low pH conditions, the native heme cofactor is removed from the heme pocket. After reconstitution at physiological pH conditions, hemoprotein assemblies are obtained via artificial heme-heme pocket interactions. b) Heterotypic co-assembly of dimerized apomyoglobin (apo-MbA125C)2 and streptavidin (Sav) is achieved using a bis(biotin)-heme bifunctional ligand. Adapted with permission from Ref.251. Copyright 2012 RSC.
