Figure 1: Summary of immune pathways of recognition and rejection following implantation of allogenic pancreatic islets.
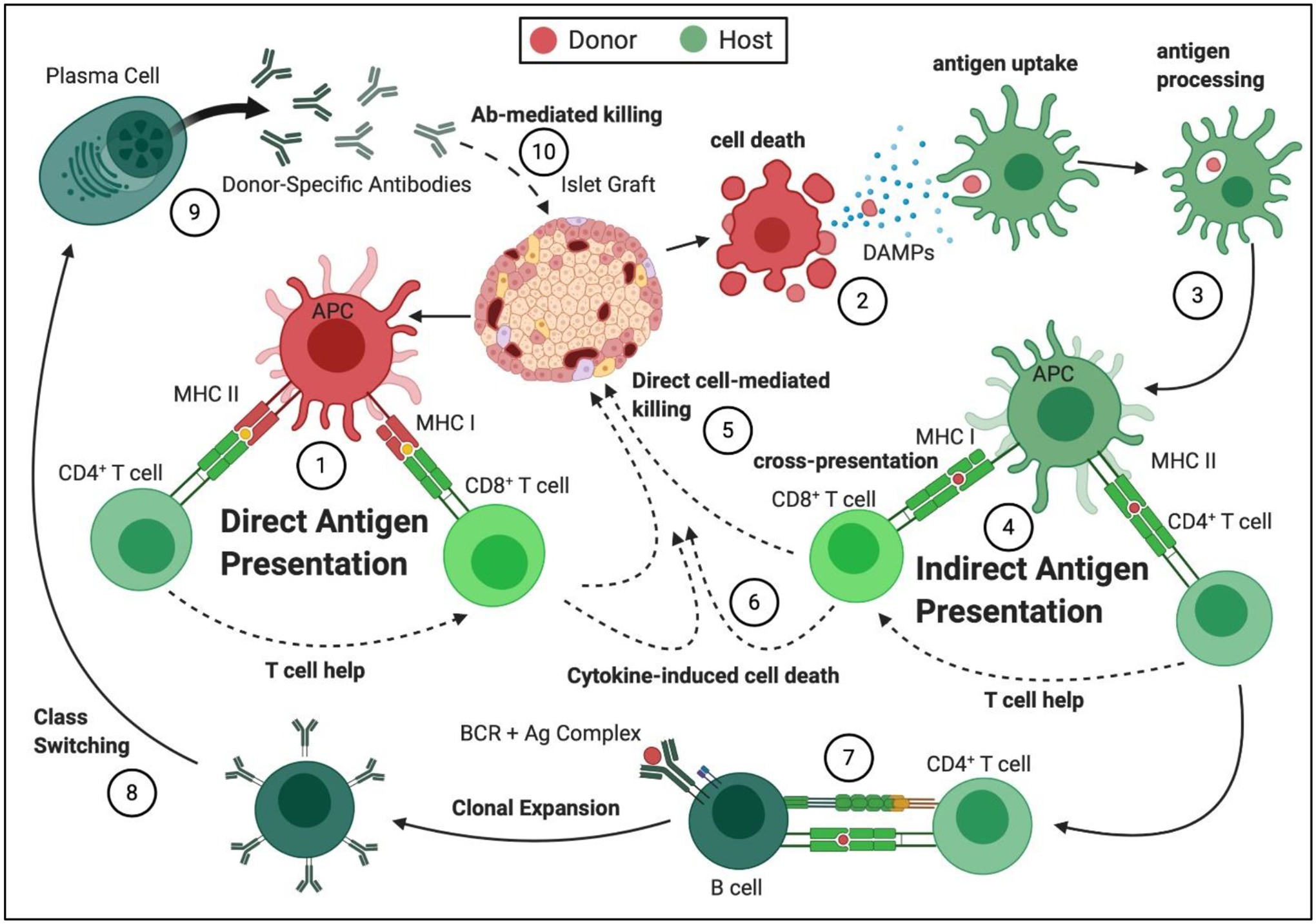
(1) Direct antigen presentation: Donor islet antigen presenting cells (APCs) present antigens to the host T lymphocytes through MHC Class I for CD8+ T cells and MHC Class II for CD4+ T cells. CD4+ T cells become activated and provide help to the CD8+ T cells, resulting in their clonal expansion and activation. Auto-antigens can also be recognized in this manner, when donor and host MHC match. (2) Donor cell death: Dying transplanted cells shed antigen and release danger-associated molecular patterns (DAMPs). (3) Antigen uptake and processing: The presence of DAMPs during antigen uptake and processing by APCs initiate and perpetuate effector signals in APCs (e.g. elevated MHCII expression). (4) Indirect Antigen Presentation: Processed host allo- or autoantigens present to host T cells in context of the self-MHC on the host APCs, resulting in T cell clonal expansion and activation. (5) Direct Cell-Mediated Killing: Cytotoxic T lymphocytes (CTLs) migrate to the implant site and initiate cell-cell interactions with the donor cells, resulting in destruction of the donor cell graft. (6) Cytokine-Induced Cell Death: Activated T cells and innate immune cells produce pro-inflammatory cytokines, which damage cells within the graft site. (7) CD4+ T cell- Mediated B cell Activation: CD4+ T cells, activated through the indirect pathway, interact with antigen-specific B cells through CD40-CD40L and provide Signal 2 for B cell activation, initiating their proliferation and differentiation. (8) Antibody Class Switching: CD4+ T cells activated through the indirect pathway allow for class switching of the produced antibodies to isotype IgG, which is associated with antibody-mediated rejection (AMR). (9) Release of Donor-Specific Antibodies (DSA): Production of DSAs by plasma cells results in acute and chronic (10) Antibody-Mediated Graft Destruction, which involves antibody binding to donor cell surface antigens, complement activation, and graft destruction.
