Figure 1.
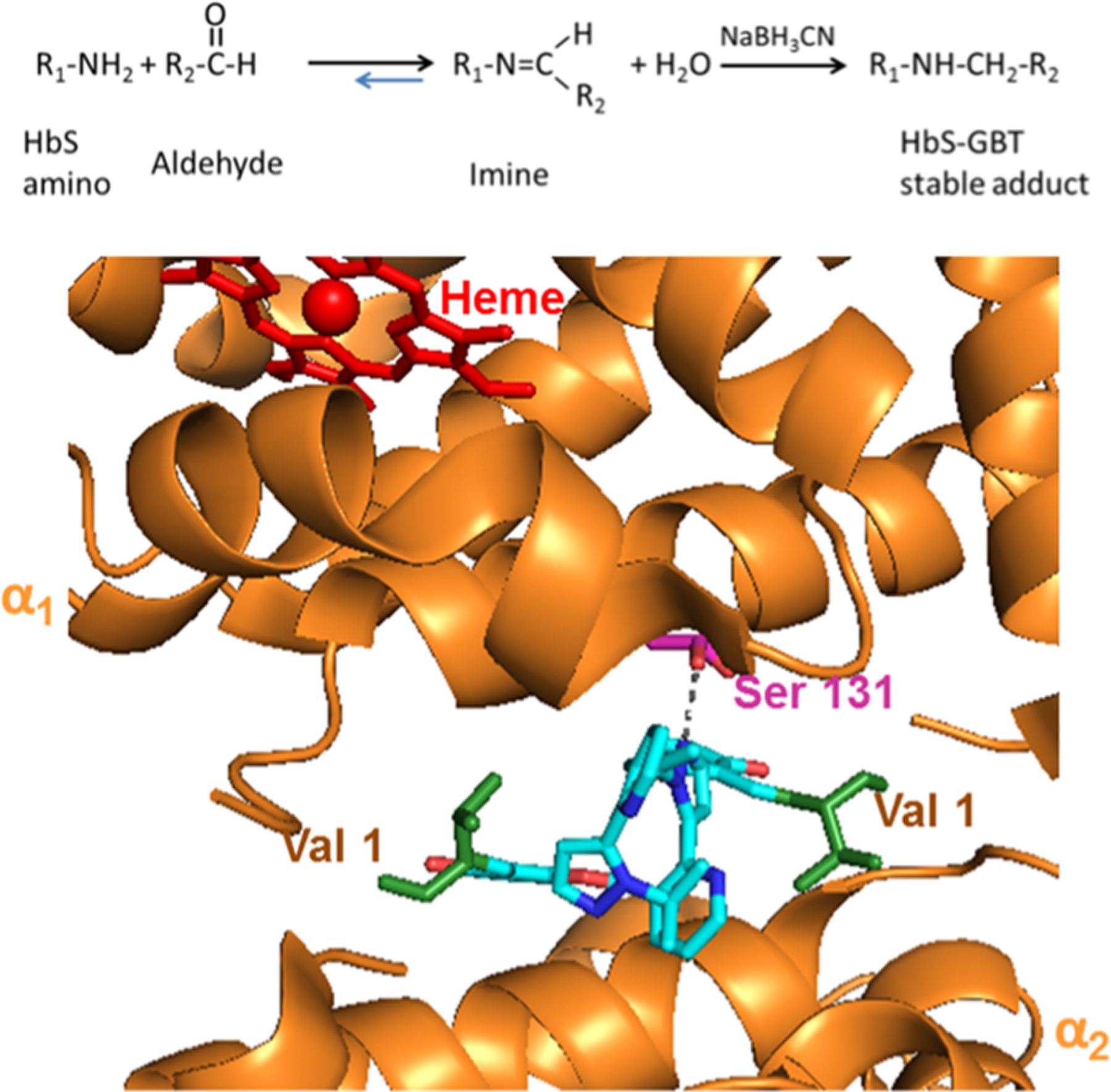
Scheme (top panel) represents the chemical reaction showing how GBT440 modifies HbS primary amines to form a reversible Schiff base imine. Na BH3CN converts the reversible Schiff base imine into a covalent HbS-GBT440 stable adduct that can easily be detected and characterized using reverse phase HPLC and mass spectrometry. The panel below represents the structure of GBT440 (cyan) complexed with HbS (PDB 5E83). The α subunits are represented by orange, the heme by red, and corresponding N-terminal valines by green. The image was made using PyMOL software.
